You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000265_02895
You are here: Home > Sequence: MGYG000000265_02895
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides nordii | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides nordii | |||||||||||
| CAZyme ID | MGYG000000265_02895 | |||||||||||
| CAZy Family | GH36 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 2382; End: 7718 Strand: - | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd14791 | GH36 | 7.50e-14 | 308 | 564 | 3 | 278 | glycosyl hydrolase family 36 (GH36). GH36 enzymes occur in prokaryotes, eukaryotes, and archaea with a wide range of hydrolytic activities, including alpha-galactosidase, alpha-N-acetylgalactosaminidase, stachyose synthase, and raffinose synthase. All GH36 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH36 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| pfam13517 | VCBS | 2.27e-11 | 949 | 1010 | 1 | 61 | Repeat domain in Vibrio, Colwellia, Bradyrhizobium and Shewanella. This domain of about 100 residues is found in multiple (up to 35) copies in long proteins from several species of Vibrio, Colwellia, Bradyrhizobium, and Shewanella (hence the name VCBS) and in smaller copy numbers in proteins from several other bacteria. The large protein size and repeat copy numbers, species distribution, and suggested activities of several member proteins suggests a role for this domain in adhesion (TIGR). |
| cd14792 | GH27 | 1.47e-10 | 308 | 592 | 2 | 268 | glycosyl hydrolase family 27 (GH27). GH27 enzymes occur in eukaryotes, prokaryotes, and archaea with a wide range of hydrolytic activities, including alpha-glucosidase (glucoamylase and sucrase-isomaltase), alpha-N-acetylgalactosaminidase, and 3-alpha-isomalto-dextranase. All GH27 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH27 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| pfam13517 | VCBS | 1.71e-09 | 1453 | 1512 | 1 | 61 | Repeat domain in Vibrio, Colwellia, Bradyrhizobium and Shewanella. This domain of about 100 residues is found in multiple (up to 35) copies in long proteins from several species of Vibrio, Colwellia, Bradyrhizobium, and Shewanella (hence the name VCBS) and in smaller copy numbers in proteins from several other bacteria. The large protein size and repeat copy numbers, species distribution, and suggested activities of several member proteins suggests a role for this domain in adhesion (TIGR). |
| pfam17801 | Melibiase_C | 1.92e-08 | 624 | 684 | 15 | 74 | Alpha galactosidase C-terminal beta sandwich domain. This domain is found at the C-terminus of alpha galactosidase enzymes. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AND18605.1 | 1.81e-158 | 26 | 684 | 32 | 673 |
| AII66839.1 | 1.81e-158 | 26 | 684 | 32 | 673 |
| ALA72670.1 | 1.81e-158 | 26 | 684 | 32 | 673 |
| QJR53686.1 | 1.81e-158 | 26 | 684 | 32 | 673 |
| QJR61515.1 | 1.81e-158 | 26 | 684 | 32 | 673 |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
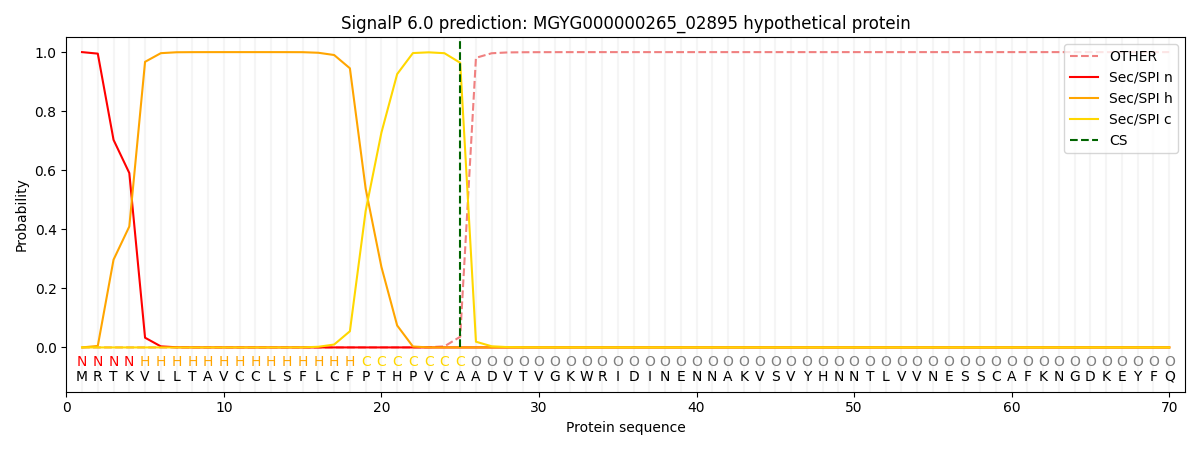
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000278 | 0.998639 | 0.000555 | 0.000190 | 0.000165 | 0.000150 |
