You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002783_00108
You are here: Home > Sequence: MGYG000002783_00108
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Prevotellamassilia sp900541335 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotellamassilia; Prevotellamassilia sp900541335 | |||||||||||
| CAZyme ID | MGYG000002783_00108 | |||||||||||
| CAZy Family | GH33 | |||||||||||
| CAZyme Description | Sialidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 133918; End: 135123 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH33 | 20 | 386 | 2.2e-47 | 0.9707602339181286 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd15482 | Sialidase_non-viral | 1.08e-62 | 21 | 389 | 1 | 339 | Non-viral sialidases. Sialidases or neuraminidases function to bind and hydrolyze terminal sialic acid residues from various glycoconjugates, they play vital roles in pathogenesis, bacterial nutrition and cellular interactions. They have a six-bladed, beta-propeller fold with the non-viral sialidases containing 2-5 Asp-box motifs (most commonly Ser/Thr-X-Asp-[X]-Gly-X-Thr- Trp/Phe). This CD includes eubacterial and eukaryotic sialidases. |
| COG4409 | NanH | 2.12e-04 | 18 | 127 | 258 | 387 | Neuraminidase (sialidase) [Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QFQ12979.1 | 1.43e-184 | 12 | 398 | 12 | 397 |
| EFC70822.1 | 1.76e-159 | 1 | 398 | 1 | 402 |
| ALO48969.1 | 4.90e-118 | 23 | 398 | 29 | 399 |
| QYR10380.1 | 2.12e-112 | 10 | 398 | 13 | 395 |
| QFQ13500.1 | 2.10e-88 | 28 | 401 | 632 | 1010 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7LBU_A | 3.56e-13 | 31 | 247 | 91 | 303 | ChainA, Exo-alpha-sialidase [Cutibacterium acnes],7LBV_A Chain A, Exo-alpha-sialidase [Cutibacterium acnes] |
| 2BF6_A | 1.95e-11 | 23 | 396 | 12 | 449 | AtomicResolution Structure of the bacterial sialidase NanI from Clostridium perfringens in complex with alpha-Sialic Acid (Neu5Ac). [Clostridium perfringens] |
| 2VK5_A | 1.97e-11 | 23 | 396 | 12 | 449 | TheStructure Of Clostridium Perfringens Nani Sialidase And Its Catalytic Intermediates [Clostridium perfringens],2VK6_A The Structure Of Clostridium Perfringens Nani Sialidase And Its Catalytic Intermediates [Clostridium perfringens],2VK7_A The Structure Of Clostridium Perfringens Nani Sialidase And Its Catalytic Intermediates [Clostridium perfringens],2VK7_B The Structure Of Clostridium Perfringens Nani Sialidase And Its Catalytic Intermediates [Clostridium perfringens] |
| 5TSP_A | 1.12e-10 | 23 | 396 | 13 | 450 | Crystalstructure of the catalytic domain of Clostridium perfringens neuraminidase (NanI) in complex with a CHES [Clostridium perfringens ATCC 13124],5TSP_B Crystal structure of the catalytic domain of Clostridium perfringens neuraminidase (NanI) in complex with a CHES [Clostridium perfringens ATCC 13124] |
| 7MHU_A | 9.80e-10 | 17 | 252 | 33 | 266 | ChainA, Exo-alpha-sialidase [Bacteroides acidifaciens],7MHU_B Chain B, Exo-alpha-sialidase [Bacteroides acidifaciens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q02834 | 1.32e-08 | 31 | 375 | 63 | 386 | Sialidase OS=Micromonospora viridifaciens OX=1881 GN=nedA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
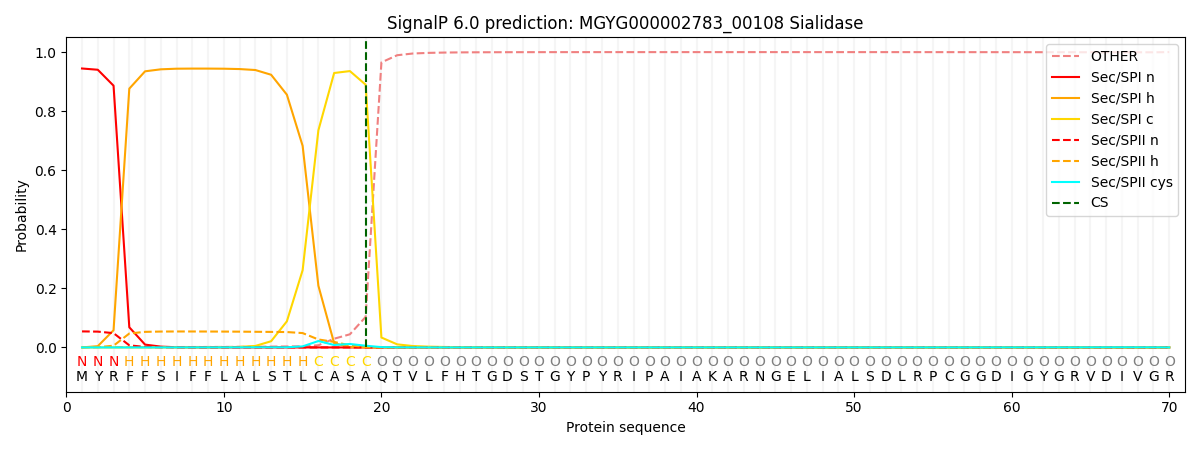
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.002743 | 0.938671 | 0.057690 | 0.000330 | 0.000275 | 0.000278 |
