You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000608_00802
You are here: Home > Sequence: MGYG000000608_00802
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Verrucomicrobiota; Lentisphaeria; Victivallales; Victivallaceae; UMGS1518; | |||||||||||
| CAZyme ID | MGYG000000608_00802 | |||||||||||
| CAZy Family | GH20 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 1501; End: 3738 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH20 | 187 | 511 | 4e-109 | 0.973293768545994 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd06563 | GH20_chitobiase-like | 4.23e-174 | 192 | 524 | 1 | 357 | The chitobiase of Serratia marcescens is a beta-N-1,4-acetylhexosaminidase with a glycosyl hydrolase family 20 (GH20) domain that hydrolyzes the beta-1,4-glycosidic linkages in oligomers derived from chitin. Chitin is degraded by a two step process: i) a chitinase hydrolyzes the chitin to oligosaccharides and disaccharides such as di-N-acetyl-D-glucosamine and chitobiose, ii) chitobiase then further degrades these oligomers into monomers. This GH20 domain family includes an N-acetylglucosamidase (GlcNAcase A) from Pseudoalteromonas piscicida and an N-acetylhexosaminidase (SpHex) from Streptomyces plicatus. SpHex lacks the C-terminal PKD (polycystic kidney disease I)-like domain found in the chitobiases. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. |
| pfam00728 | Glyco_hydro_20 | 4.98e-145 | 192 | 511 | 1 | 345 | Glycosyl hydrolase family 20, catalytic domain. This domain has a TIM barrel fold. |
| COG3525 | Chb | 4.06e-107 | 23 | 539 | 131 | 643 | N-acetyl-beta-hexosaminidase [Carbohydrate transport and metabolism]. |
| cd06570 | GH20_chitobiase-like_1 | 1.22e-95 | 192 | 524 | 1 | 311 | A functionally uncharacterized subgroup of the Glycosyl hydrolase family 20 (GH20) catalytic domain found in proteins similar to the chitobiase of Serratia marcescens, a beta-N-1,4-acetylhexosaminidase that hydrolyzes the beta-1,4-glycosidic linkages in oligomers derived from chitin. Chitin is degraded by a two step process: i) a chitinase hydrolyzes the chitin to oligosaccharides and disaccharides such as di-N-acetyl-D-glucosamine and chitobiose, ii) chitobiase then further degrades these oligomers into monomers. This subgroup lacks the C-terminal PKD (polycystic kidney disease I)-like domain found in the chitobiases. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. |
| cd06562 | GH20_HexA_HexB-like | 3.64e-87 | 192 | 530 | 1 | 343 | Beta-N-acetylhexosaminidases catalyze the removal of beta-1,4-linked N-acetyl-D-hexosamine residues from the non-reducing ends of N-acetyl-beta-D-hexosaminides including N-acetylglucosides and N-acetylgalactosides. The hexA and hexB genes encode the alpha- and beta-subunits of the two major beta-N-acetylhexosaminidase isoenzymes, N-acetyl-beta-D-hexosaminidase A (HexA) and beta-N-acetylhexosaminidase B (HexB). Both the alpha and the beta catalytic subunits have a TIM-barrel fold and belong to the glycosyl hydrolase family 20 (GH20). The HexA enzyme is a heterodimer containing one alpha and one beta subunit while the HexB enzyme is a homodimer containing two beta-subunits. Hexosaminidase mutations cause an inability to properly hydrolyze certain sphingolipids which accumulate in lysosomes within the brain, resulting in the lipid storage disorders Tay-Sachs and Sandhoff. Mutations in the alpha subunit cause in a deficiency in the HexA enzyme and result in Tay-Sachs, mutations in the beta-subunit cause in a deficiency in both HexA and HexB enzymes and result in Sandhoff disease. In both disorders GM(2) gangliosides accumulate in lysosomes. The GH20 hexosaminidases are thought to act via a catalytic mechanism in which the catalytic nucleophile is not provided by solvent or the enzyme, but by the substrate itself. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BBE18475.1 | 1.71e-132 | 22 | 597 | 27 | 604 |
| AHJ98435.1 | 1.17e-131 | 14 | 745 | 52 | 815 |
| AKD05472.1 | 1.80e-130 | 31 | 612 | 18 | 607 |
| SDT55220.1 | 3.15e-130 | 7 | 619 | 7 | 626 |
| QIU95644.1 | 7.08e-129 | 23 | 733 | 26 | 740 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6Q63_A | 3.53e-126 | 23 | 704 | 26 | 713 | BT0459[Bacteroides thetaiotaomicron],6Q63_B BT0459 [Bacteroides thetaiotaomicron],6Q63_C BT0459 [Bacteroides thetaiotaomicron] |
| 3RCN_A | 1.06e-85 | 39 | 524 | 12 | 498 | CrystalStructure of Beta-N-Acetylhexosaminidase from Arthrobacter aurescens [Paenarthrobacter aurescens TC1] |
| 7CBN_A | 4.20e-80 | 107 | 533 | 77 | 505 | Crystalstructure of beta-N-acetylhexosaminidase Am0868 from Akkermansia muciniphila [Akkermansia muciniphila ATCC BAA-835],7CBO_A Crystal structure of beta-N-acetylhexosaminidase Am0868 from Akkermansia muciniphila in complex with GlcNAc [Akkermansia muciniphila ATCC BAA-835] |
| 6EZR_A | 4.51e-78 | 110 | 541 | 211 | 640 | Crystalstructure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi [Vibrio harveyi],6EZR_B Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi [Vibrio harveyi],6EZS_A Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi in complex with N-acetylglucosamine [Vibrio harveyi],6EZS_B Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase from Vibrio harveyi in complex with N-acetylglucosamine [Vibrio harveyi],6K35_A Crystal structure of GH20 exo beta-N-acetylglucosaminidase from Vibrio harveyi in complex with NAG-thiazoline [Vibrio harveyi],6K35_B Crystal structure of GH20 exo beta-N-acetylglucosaminidase from Vibrio harveyi in complex with NAG-thiazoline [Vibrio harveyi] |
| 6EZT_A | 5.88e-77 | 110 | 541 | 208 | 637 | Crystalstructure of GH20 Exo beta-N-Acetylglucosaminidase D437A inactive mutant from Vibrio harveyi [Vibrio harveyi],6EZT_B Crystal structure of GH20 Exo beta-N-Acetylglucosaminidase D437A inactive mutant from Vibrio harveyi [Vibrio harveyi] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P49008 | 7.85e-110 | 36 | 626 | 44 | 624 | Beta-hexosaminidase OS=Porphyromonas gingivalis (strain ATCC BAA-308 / W83) OX=242619 GN=nahA PE=3 SV=2 |
| B2UQG6 | 3.04e-79 | 107 | 533 | 96 | 524 | Beta-hexosaminidase Amuc_0868 OS=Akkermansia muciniphila (strain ATCC BAA-835 / DSM 22959 / JCM 33894 / BCRC 81048 / CCUG 64013 / CIP 107961 / Muc) OX=349741 GN=Amuc_0868 PE=1 SV=1 |
| P96155 | 9.86e-71 | 110 | 505 | 210 | 601 | Beta-hexosaminidase OS=Vibrio furnissii OX=29494 GN=exoI PE=1 SV=1 |
| Q7WUL4 | 6.17e-54 | 26 | 536 | 4 | 475 | Beta-N-acetylhexosaminidase OS=Cellulomonas fimi OX=1708 GN=hex20 PE=1 SV=1 |
| P07686 | 1.02e-49 | 161 | 526 | 173 | 531 | Beta-hexosaminidase subunit beta OS=Homo sapiens OX=9606 GN=HEXB PE=1 SV=4 |
SignalP and Lipop Annotations help
This protein is predicted as SP
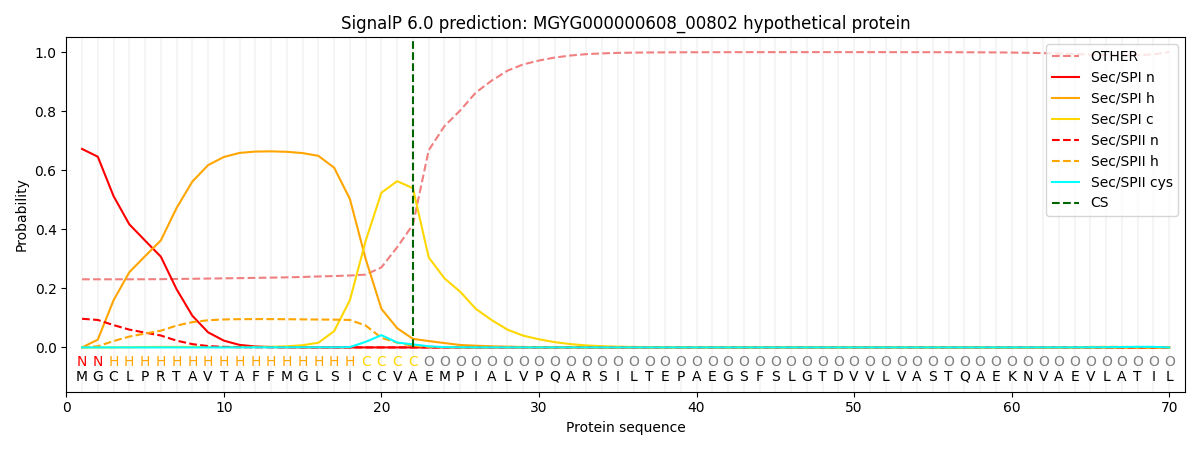
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.252975 | 0.644591 | 0.099434 | 0.001148 | 0.000712 | 0.001116 |

