You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000403_00006
You are here: Home > Sequence: MGYG000000403_00006
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
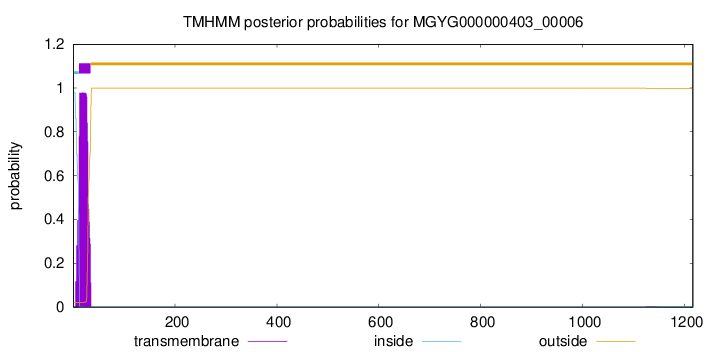
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Monoglobales_A; UBA1381; UBA4716; | |||||||||||
| CAZyme ID | MGYG000000403_00006 | |||||||||||
| CAZy Family | PL21 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 7679; End: 11329 Strand: + | |||||||||||
Full Sequence Download help
| MTKKYITRRF IAAVLSAAML CGVAFSAFAD GINYGDKVEN PSIPLLLDEK NTVKNGNLVF | 60 |
| EAEDMKLASD MVIGQNENAS GGKYITGATG TTKGSNSGNK NPSYSMNIKV EKSGPYSIWI | 120 |
| RARITHNGAD SINLAVNSKH YEYFALPIEP DWQWINLKST SLPAGMVAIN FRYAEPPAWI | 180 |
| DKVLITADTE FVPKEMNDMP NAVEMGDVIY PEPEIKPISG HPRLFLTKDY IPELVKKREA | 240 |
| PELAKAWKVI DDWADLYIDA KLPERDSGNF SENILMRIQA KALKYVMDDS DKELADEVIR | 300 |
| LAKDYLKTVS FSNGGDVTRN RGDVMVMAAI VYDWCYDRMT DEDKQYFIKR FKELALEKEV | 360 |
| GYPPTNLSSV TSHTGEGEIM RDLLSAGIAC YDEDTEIYNL AGGRFFSEMT EPRKMYYDAG | 420 |
| RHPEGSSYGP YRFQWEAFAA VIFDRMGIHN VFGENMKNVN YHWLYDMRPD GFRMKEGDEP | 480 |
| TFSAANYFYY MGTERRSAMI VGSLFKDPYI RGRYLKESAS GGYVNDYFWT VLFDDPEVGA | 540 |
| KEPYDLPLTE FNGWPLSGMV ARTSWQDGLD SPAVVASVTM KAINTGDHMH RDAGAFSIYY | 600 |
| KGALAIDSGE YQGQSGGYGY PHDMNYNKKS IAHNVVTVYD PDEKDNQVLS STNDGGQRYP | 660 |
| RTSGMAKTLE ELTADDAILA EEKAHSAGPN DKTPEYSYLK GDLTKAYTDK ITDYKRSFVF | 720 |
| LNLDDEDYPA AAVVFDKVDS KKKEFKKKWL LHSIEEPDVE GDTTTIARTE YGFDGKLVNK | 780 |
| TLLPETKNLS IEKIGGSGKE FWVENQNFAN APKANGTEQG AWRIEVSPKN EAESDTFLNA | 840 |
| MYVTDNSSEL PQLEAEKIET DDYVGALIKD RAVLFAKDSE PKNNEIKISC GKETAFLITD | 900 |
| VAEGTWQISG NGLNIFANSE KDGNCLYFRV PAGEYTVTPT EAHEETAENV PEMQVKTSGD | 960 |
| FTVWDEKARQ FLYAESKLID GVPYVQAKRI FEKYDSAVKW DGGTGEITIS SGGKTIRIKS | 1020 |
| GSTAAVMNKE EIQLQYAPVI IDGVTYVYPC DFTELTGKEF SFDETAKMLL VRDKKSEPVP | 1080 |
| IKGIDEENVI KPVTVACSDS DTNIADNASD LDLTTRWSAN GDGQWLMYDM GEVVPISRVA | 1140 |
| LAFYLGSQRQ TYFDIQLSND AKTFTTVYSG ASSGKTENPE FYPINYSARY IRFMGHGTAA | 1200 |
| NRTGWNSVTE MTVLKK | 1216 |
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL21 | 573 | 642 | 1.6e-25 | 0.9583333333333334 |
| CBM32 | 1098 | 1211 | 4.9e-19 | 0.8951612903225806 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam00754 | F5_F8_type_C | 7.11e-14 | 1098 | 1211 | 7 | 127 | F5/8 type C domain. This domain is also known as the discoidin (DS) domain family. |
| pfam07833 | Cu_amine_oxidN1 | 1.88e-13 | 979 | 1071 | 1 | 93 | Copper amine oxidase N-terminal domain. Copper amine oxidases catalyze the oxidative deamination of primary amines to the corresponding aldehydes, while reducing molecular oxygen to hydrogen peroxide. These enzymes are dimers of identical subunits, each comprising four domains. The N-terminal domain, which is absent in some amine oxidases, consists of a five-stranded antiparallel beta sheet twisted around an alpha helix. The D1 domains from the two subunits comprise the 'stalk' of the mushroom-shaped dimer, and interact with each other but do not pack tightly against each other. |
| pfam18675 | HepII_C | 6.76e-09 | 858 | 938 | 2 | 87 | Heparinase II C-terminal domain. Heparinase II (HepII) is an 85-kDa dimeric enzyme that depolymerizes both heparin and heparan sulfate glycosaminoglycans. The protein is composed of three domains: an N-terminal alpha-helical domain, a central two-layered beta-sheet domain, and a C-terminal domain forming a two-layered beta-sheet. The C-terminal domain contains nine beta-strands packed together in a manner resembling a beta-barrel. |
| cd00057 | FA58C | 3.98e-07 | 1093 | 1211 | 14 | 137 | Substituted updates: Jan 31, 2002 |
| cd04086 | CBM35_mannanase-like | 1.39e-04 | 59 | 165 | 2 | 98 | Carbohydrate Binding Module 35 (CBM35); appended to several carbohydrate binding enzymes, including several glycoside hydrolase (GH) family 26 mannanase domains. This family includes carbohydrate binding module 35 (CBM35) domains that are appended to several carbohydrate binding enzymes, including periplasmic component of ABC-type sugar transport system involved in carbohydrate transport and metabolism, and several glycoside hydrolase (GH) domains, including GH26. These CBM6s are non-catalytic carbohydrate binding domains that facilitate the strong binding of the GH catalytic modules with their dedicated, insoluble substrates. Examples of proteins having CMB35s belonging to this family are mannanase A from Clostridium thermocellum (GH26), Man26B from Paenibacillus sp. BME-14 (GH26), and the multifunctional Cel44C-Man26A from Paenibacillus polymyxa GS01 (which has two GH domains, GH44 and GH26). GH26 mainly includes mannan endo-1,4-beta-mannosidase which hydrolyzes 1,4-beta-D-linkages in mannans, galacto-mannans, glucomannans, and galactoglucomannans, but displays little activity towards other plant cell wall polysaccharides. A few proteins belonging to this family have additional CBM3 domains; these CBM3s are not found in the CBM6-CBM35-CBM36_like superfamily. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BBH20141.1 | 1.59e-202 | 210 | 1209 | 228 | 1219 |
| QHW00380.1 | 6.24e-169 | 217 | 938 | 67 | 799 |
| ACU04612.1 | 4.20e-167 | 217 | 935 | 44 | 768 |
| ABJ17170.1 | 1.16e-166 | 217 | 935 | 44 | 768 |
| AAB18277.1 | 1.16e-166 | 217 | 935 | 44 | 768 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2FUQ_A | 7.16e-169 | 217 | 935 | 19 | 743 | ChainA, heparinase II protein [Pedobacter heparinus],2FUQ_B Chain B, heparinase II protein [Pedobacter heparinus] |
| 3E80_A | 7.61e-169 | 217 | 935 | 21 | 745 | Structureof Heparinase II complexed with heparan sulfate degradation disaccharide product [Pedobacter heparinus],3E80_B Structure of Heparinase II complexed with heparan sulfate degradation disaccharide product [Pedobacter heparinus],3E80_C Structure of Heparinase II complexed with heparan sulfate degradation disaccharide product [Pedobacter heparinus] |
| 3E7J_A | 4.78e-166 | 217 | 935 | 21 | 745 | ChainA, Heparinase II protein [Pedobacter heparinus],3E7J_B Chain B, Heparinase II protein [Pedobacter heparinus] |
| 2FUT_A | 2.89e-163 | 217 | 935 | 20 | 744 | ChainA, heparinase II protein [Pedobacter heparinus],2FUT_B Chain B, heparinase II protein [Pedobacter heparinus] |
| 5ZU6_A | 1.58e-24 | 1087 | 1214 | 29 | 157 | ACBM32 derived from alginate lyase B (AlyB-OU02) [Vibrio] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| C6XZB6 | 8.41e-168 | 217 | 935 | 44 | 768 | Heparin and heparin-sulfate lyase OS=Pedobacter heparinus (strain ATCC 13125 / DSM 2366 / CIP 104194 / JCM 7457 / NBRC 12017 / NCIMB 9290 / NRRL B-14731 / HIM 762-3) OX=485917 GN=hepB PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
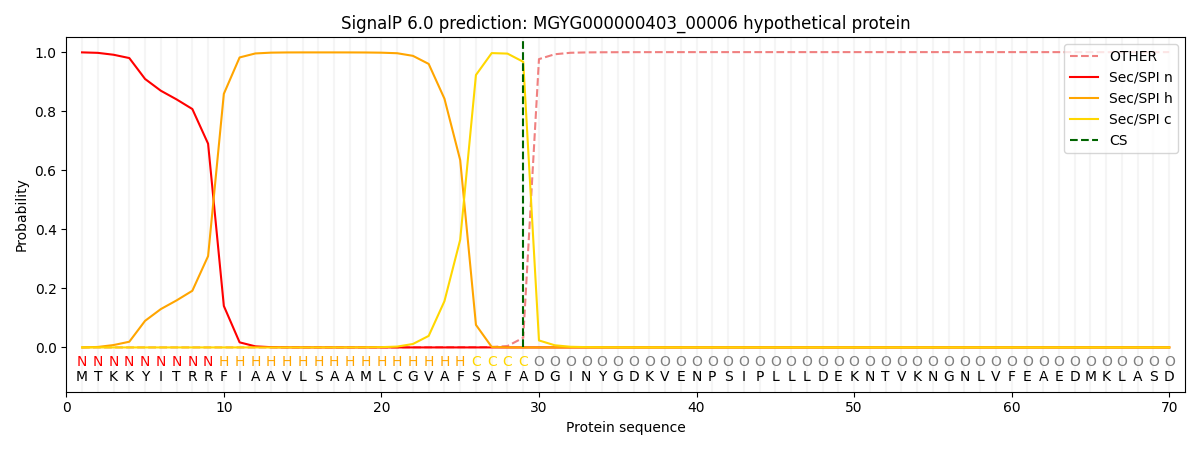
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000475 | 0.997311 | 0.001368 | 0.000412 | 0.000226 | 0.000171 |