You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000002781_00247
You are here: Home > Sequence: MGYG000002781_00247
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | UMGS775 sp900545325 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia_A; Christensenellales; Borkfalkiaceae; UMGS775; UMGS775 sp900545325 | |||||||||||
| CAZyme ID | MGYG000002781_00247 | |||||||||||
| CAZy Family | GH32 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 27919; End: 30387 Strand: + | |||||||||||
Full Sequence Download help
| MKRGFFKKAG TCLLACALAF GAAGTAGCAE KGAVSDGLAA YYSFDEGEGS YAKEKVSGKT | 60 |
| HEIYNVYAAS NQQNLMKPSG EPLWREGVKG SCLYMDGVSN YIEDDSFKNL STGALTLSAW | 120 |
| VAPRVFENNF KTEGLTCIAG KGDVGLQEGW LLGYGYLGTW GLKVALQDPE TGNTFTAAFY | 180 |
| DPVNYLPLYE WSHVAASYDA SSGRVCLYLN GELVYEQIYN EYAGCEIVPS SDPLRIGKYI | 240 |
| DPASVYGIDC NLVAGLLDEV YIYETELSHA QVKALYEDKD AAGHPACDYA DVMLDSSVYE | 300 |
| GDRYRPQYHA IPPGMWMNEP HAAFYYNGYY HLFYQSNPNG PYWAQIRWSH WVSEDMVHWK | 360 |
| YVKEAVVPTK DICPDGVWTG GAVIGPDGTP WLVITAGTTT TTYSGQNIAF AHCADPTDPY | 420 |
| LEEWVVEDKV AITQEAGMGK INQFRDPFIW QDEGVYYMMV GSGKEGASSG GALFFTSSDM | 480 |
| RDWEYHGYVF ESAAEEAGIH WECIQLLPVS TKDGSQQKYV FFLTPQYADD SRTVECYYWI | 540 |
| GTFDKEAKKF VPDAGYESPR LLDYGYGIFT GQTGFTYLTE EDAAAGKTYT QGRTVLFAIA | 600 |
| QGKEAGTTHN YYSGWAHNAS LPVELFLSED GHSLNVEPIP ELTALRQETL YENGASMSAE | 660 |
| QANAAIGQVR GDLLEIRAKI TVSETASNYK AGLYVRYNPY NNSLNQTERT ALVFAENGVY | 720 |
| VDRSQTSLYV QGLKNSHVWE NTEKEHDVIV YLDRSMLEIY IDGKVSFTSR MYPKYEDSDY | 780 |
| LSFFAEGCVL SVSDLTIYRL GSAYSDTVTP AYYGNTGSIK DA | 822 |
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH32 | 309 | 634 | 5.2e-66 | 0.9829351535836177 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| smart00640 | Glyco_32 | 2.21e-84 | 309 | 763 | 1 | 436 | Glycosyl hydrolases family 32. |
| cd08996 | GH32_FFase | 6.10e-84 | 316 | 627 | 2 | 281 | Glycosyl hydrolase family 32, beta-fructosidases. Glycosyl hydrolase family GH32 cleaves sucrose into fructose and glucose via beta-fructofuranosidase activity, producing invert sugar that is a mixture of dextrorotatory D-glucose and levorotatory D-fructose, thus named invertase (EC 3.2.1.26). This family also contains other fructofuranosidases such as inulinase (EC 3.2.1.7), exo-inulinase (EC 3.2.1.80), levanase (EC 3.2.1.65), and transfructosidases such sucrose:sucrose 1-fructosyltransferase (EC 2.4.1.99), fructan:fructan 1-fructosyltransferase (EC 2.4.1.100), sucrose:fructan 6-fructosyltransferase (EC 2.4.1.10), fructan:fructan 6G-fructosyltransferase (EC 2.4.1.243) and levan fructosyltransferases (EC 2.4.1.-). These retaining enzymes (i.e. they retain the configuration at anomeric carbon atom of the substrate) catalyze hydrolysis in two steps involving a covalent glycosyl enzyme intermediate: an aspartate located close to the N-terminus acts as the catalytic nucleophile and a glutamate acts as the general acid/base; a conserved aspartate residue in the Arg-Asp-Pro (RDP) motif stabilizes the transition state. These enzymes are predicted to display a 5-fold beta-propeller fold as found for GH43 and CH68. The breakdown of sucrose is widely used as a carbon or energy source by bacteria, fungi, and plants. Invertase is used commercially in the confectionery industry, since fructose has a sweeter taste than sucrose and a lower tendency to crystallize. A common structural feature of all these enzymes is a 5-bladed beta-propeller domain, similar to GH43, that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| pfam00251 | Glyco_hydro_32N | 3.18e-60 | 309 | 638 | 1 | 308 | Glycosyl hydrolases family 32 N-terminal domain. This domain corresponds to the N-terminal domain of glycosyl hydrolase family 32 which forms a five bladed beta propeller structure. |
| COG1621 | SacC | 4.10e-54 | 294 | 802 | 19 | 486 | Sucrose-6-phosphate hydrolase SacC, GH32 family [Carbohydrate transport and metabolism]. |
| cd18624 | GH32_Fruct1-like | 5.16e-50 | 316 | 569 | 2 | 250 | glycoside hydrolase family 32 protein such as Arabidopsis thaliana cell-wall invertase 1 (AtBFruct1;Fruct1;AtcwINV1;At3g13790). This subfamily of glycosyl hydrolase family GH32 includes fructan beta-(2,1)-fructosidase and fructan 1-exohydrolase IIa (1-FEH IIa, EC 3.2.1.153), cell-wall invertase 1 (EC 3.2.1.26), sucrose:fructan 6-fructosyltransferase (6-Sst/6-Dft, EC 2.4.1.10), and levan fructosyltransferases (EC 2.4.1.-) among others. This enzyme cleaves sucrose into fructose and glucose via beta-fructofuranosidase activity, producing invert sugar that is a mixture of dextrorotatory D-glucose and levorotatory D-fructose, thus named invertase. These retaining enzymes (i.e. they retain the configuration at anomeric carbon atom of the substrate) catalyze hydrolysis in two steps involving a covalent glycosyl enzyme intermediate: an aspartate located close to the N-terminus acts as the catalytic nucleophile and a glutamate acts as the general acid/base; a conserved aspartate residue in the Arg-Asp-Pro (RDP) motif stabilizes the transition state. The breakdown of sucrose is widely used as a carbon or energy source by bacteria, fungi, and plants. Invertase is used commercially in the confectionery industry, since fructose has a sweeter taste than sucrose and a lower tendency to crystallize. A common structural feature of all these enzymes is a 5-bladed beta-propeller domain, similar to GH43, that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ADD03331.1 | 8.38e-160 | 38 | 804 | 34 | 772 |
| ADB62922.1 | 6.13e-146 | 38 | 804 | 12 | 753 |
| AIQ31423.1 | 5.39e-141 | 37 | 804 | 9 | 758 |
| BAA24360.1 | 3.64e-140 | 24 | 812 | 544 | 1318 |
| ANC77361.1 | 7.39e-140 | 38 | 804 | 4 | 741 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1UYP_A | 6.23e-35 | 304 | 774 | 2 | 407 | Thethree-dimensional structure of beta-fructosidase (invertase) from Thermotoga maritima [Thermotoga maritima MSB8],1UYP_B The three-dimensional structure of beta-fructosidase (invertase) from Thermotoga maritima [Thermotoga maritima MSB8],1UYP_C The three-dimensional structure of beta-fructosidase (invertase) from Thermotoga maritima [Thermotoga maritima MSB8],1UYP_D The three-dimensional structure of beta-fructosidase (invertase) from Thermotoga maritima [Thermotoga maritima MSB8],1UYP_E The three-dimensional structure of beta-fructosidase (invertase) from Thermotoga maritima [Thermotoga maritima MSB8],1UYP_F The three-dimensional structure of beta-fructosidase (invertase) from Thermotoga maritima [Thermotoga maritima MSB8] |
| 1W2T_A | 1.53e-34 | 304 | 774 | 2 | 407 | beta-fructosidasefrom Thermotoga maritima in complex with raffinose [Thermotoga maritima MSB8],1W2T_B beta-fructosidase from Thermotoga maritima in complex with raffinose [Thermotoga maritima MSB8],1W2T_C beta-fructosidase from Thermotoga maritima in complex with raffinose [Thermotoga maritima MSB8],1W2T_D beta-fructosidase from Thermotoga maritima in complex with raffinose [Thermotoga maritima MSB8],1W2T_E beta-fructosidase from Thermotoga maritima in complex with raffinose [Thermotoga maritima MSB8],1W2T_F beta-fructosidase from Thermotoga maritima in complex with raffinose [Thermotoga maritima MSB8] |
| 2XQR_A | 5.26e-33 | 304 | 774 | 4 | 502 | Crystalstructure of plant cell wall invertase in complex with a specific protein inhibitor [Arabidopsis thaliana],2XQR_C Crystal structure of plant cell wall invertase in complex with a specific protein inhibitor [Arabidopsis thaliana],2XQR_E Crystal structure of plant cell wall invertase in complex with a specific protein inhibitor [Arabidopsis thaliana],2XQR_G Crystal structure of plant cell wall invertase in complex with a specific protein inhibitor [Arabidopsis thaliana],2XQR_I Crystal structure of plant cell wall invertase in complex with a specific protein inhibitor [Arabidopsis thaliana],2XQR_K Crystal structure of plant cell wall invertase in complex with a specific protein inhibitor [Arabidopsis thaliana] |
| 2AC1_A | 5.49e-33 | 304 | 774 | 8 | 506 | Crystalstructure of a cell-wall invertase from Arabidopsis thaliana [Arabidopsis thaliana] |
| 2QQU_A | 1.24e-32 | 304 | 774 | 4 | 502 | ChainA, Beta-fructofuranosidase [Arabidopsis thaliana] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| A2X5P7 | 1.45e-37 | 305 | 650 | 49 | 381 | Beta-fructofuranosidase, insoluble isoenzyme 1 OS=Oryza sativa subsp. indica OX=39946 GN=CIN1 PE=2 SV=2 |
| Q0E0P0 | 1.45e-37 | 305 | 650 | 49 | 381 | Beta-fructofuranosidase, insoluble isoenzyme 1 OS=Oryza sativa subsp. japonica OX=39947 GN=CIN1 PE=2 SV=1 |
| Q01IS7 | 3.32e-37 | 275 | 646 | 29 | 392 | Beta-fructofuranosidase, insoluble isoenzyme 2 OS=Oryza sativa subsp. indica OX=39946 GN=CIN2 PE=2 SV=2 |
| Q0JDC5 | 3.32e-37 | 275 | 646 | 29 | 392 | Beta-fructofuranosidase, insoluble isoenzyme 2 OS=Oryza sativa subsp. japonica OX=39947 GN=CIN2 PE=1 SV=1 |
| Q0JDC6 | 5.25e-37 | 289 | 646 | 31 | 385 | Beta-fructofuranosidase, insoluble isoenzyme 3 OS=Oryza sativa subsp. japonica OX=39947 GN=CIN3 PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
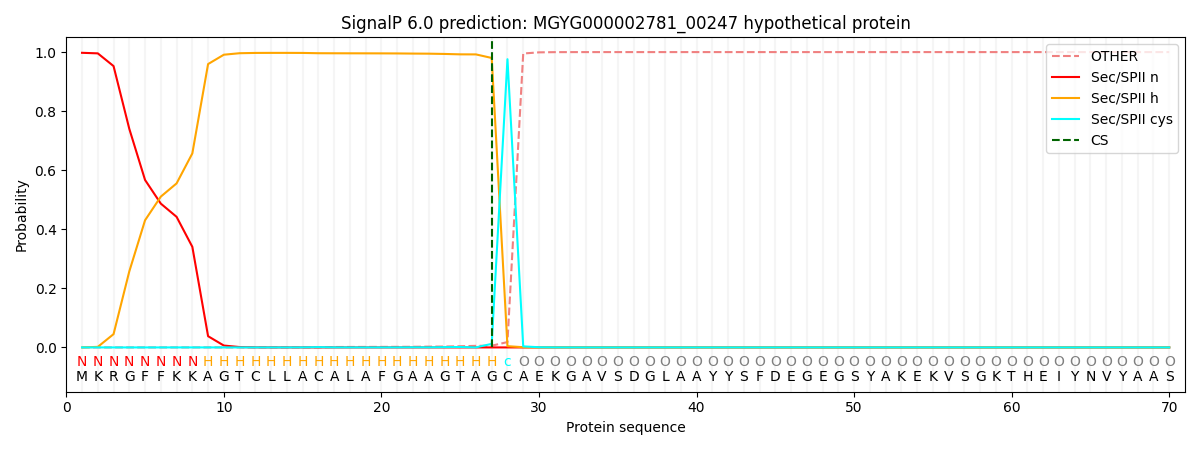
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000004 | 0.002288 | 0.997761 | 0.000001 | 0.000001 | 0.000001 |
