You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000001_01867
You are here: Home > Sequence: MGYG000000001_01867
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
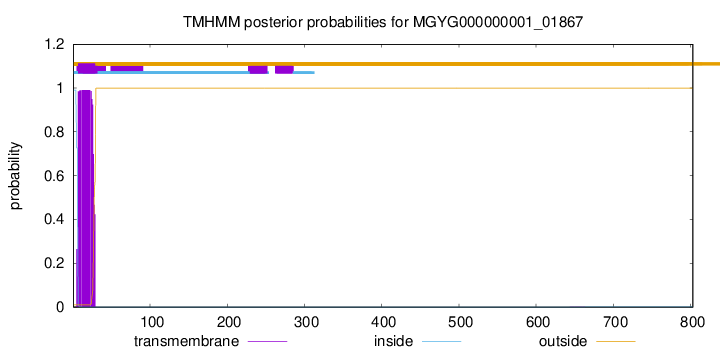
TMHMM annotations
Basic Information help
| Species | GCA-900066495 sp902362365 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Peptostreptococcales; Peptostreptococcaceae; GCA-900066495; GCA-900066495 sp902362365 | |||||||||||
| CAZyme ID | MGYG000000001_01867 | |||||||||||
| CAZy Family | CBM40 | |||||||||||
| CAZyme Description | Sialidase A | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 20135; End: 22546 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH33 | 247 | 676 | 1e-104 | 0.956140350877193 |
| CBM40 | 55 | 227 | 6.7e-48 | 0.9832402234636871 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd15482 | Sialidase_non-viral | 1.78e-93 | 246 | 677 | 1 | 339 | Non-viral sialidases. Sialidases or neuraminidases function to bind and hydrolyze terminal sialic acid residues from various glycoconjugates, they play vital roles in pathogenesis, bacterial nutrition and cellular interactions. They have a six-bladed, beta-propeller fold with the non-viral sialidases containing 2-5 Asp-box motifs (most commonly Ser/Thr-X-Asp-[X]-Gly-X-Thr- Trp/Phe). This CD includes eubacterial and eukaryotic sialidases. |
| COG4409 | NanH | 8.68e-47 | 105 | 667 | 129 | 703 | Neuraminidase (sialidase) [Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
| pfam02973 | Sialidase | 1.35e-29 | 49 | 227 | 2 | 188 | Sialidase, N-terminal domain. |
| pfam13088 | BNR_2 | 5.71e-19 | 422 | 661 | 79 | 280 | BNR repeat-like domain. This family of proteins contains BNR-like repeats suggesting these proteins may act as sialidases. |
| pfam13859 | BNR_3 | 6.20e-10 | 270 | 566 | 9 | 211 | BNR repeat-like domain. This family of proteins contains BNR-like repeats suggesting these proteins may act as sialidases. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QJA08933.1 | 0.0 | 3 | 803 | 2 | 801 |
| ATD57534.1 | 1.38e-296 | 37 | 801 | 178 | 947 |
| QBJ75069.1 | 1.38e-296 | 37 | 801 | 178 | 947 |
| ATD54786.1 | 1.38e-296 | 37 | 801 | 178 | 947 |
| SLK16343.1 | 1.38e-296 | 37 | 801 | 178 | 947 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5TSP_A | 2.39e-213 | 238 | 687 | 3 | 453 | Crystalstructure of the catalytic domain of Clostridium perfringens neuraminidase (NanI) in complex with a CHES [Clostridium perfringens ATCC 13124],5TSP_B Crystal structure of the catalytic domain of Clostridium perfringens neuraminidase (NanI) in complex with a CHES [Clostridium perfringens ATCC 13124] |
| 2VK5_A | 9.85e-213 | 238 | 687 | 2 | 452 | TheStructure Of Clostridium Perfringens Nani Sialidase And Its Catalytic Intermediates [Clostridium perfringens],2VK6_A The Structure Of Clostridium Perfringens Nani Sialidase And Its Catalytic Intermediates [Clostridium perfringens],2VK7_A The Structure Of Clostridium Perfringens Nani Sialidase And Its Catalytic Intermediates [Clostridium perfringens],2VK7_B The Structure Of Clostridium Perfringens Nani Sialidase And Its Catalytic Intermediates [Clostridium perfringens] |
| 2BF6_A | 2.51e-212 | 238 | 682 | 2 | 447 | AtomicResolution Structure of the bacterial sialidase NanI from Clostridium perfringens in complex with alpha-Sialic Acid (Neu5Ac). [Clostridium perfringens] |
| 1SLI_A | 6.85e-99 | 66 | 683 | 22 | 667 | LeechIntramolecular Trans-Sialidase Complexed With Dana [Macrobdella decora],1SLL_A Sialidase L From Leech Macrobdella Decora [Macrobdella decora],2SLI_A Leech Intramolecular Trans-Sialidase Complexed With 2,7- Anhydro-Neu5ac, The Reaction Product [Macrobdella decora],3SLI_A Leech Intramolecular Trans-Sialidase Complexed With 2,7- Anhydro-Neu5ac Prepared By Soaking With 3'-Sialyllactose [Macrobdella decora],4SLI_A Leech Intramolecular Trans-Sialidase Complexed With 2- Propenyl-Neu5ac, An Inactive Substrate Analogue [Macrobdella decora] |
| 4X6K_A | 9.42e-89 | 249 | 687 | 9 | 481 | Crystalstructure of the intramolecular trans-sialidase from Ruminococcus gnavus in complex with Siastatin B [[Ruminococcus] gnavus CC55_001C] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P29767 | 2.62e-292 | 72 | 801 | 213 | 946 | Sialidase OS=Clostridium septicum OX=1504 PE=3 SV=1 |
| P62575 | 1.42e-97 | 46 | 681 | 116 | 789 | Sialidase A OS=Streptococcus pneumoniae OX=1313 GN=nanA PE=1 SV=1 |
| P62576 | 1.42e-97 | 46 | 681 | 116 | 789 | Sialidase A OS=Streptococcus pneumoniae (strain ATCC BAA-255 / R6) OX=171101 GN=nanA PE=1 SV=1 |
| Q27701 | 2.69e-97 | 66 | 683 | 102 | 747 | Anhydrosialidase OS=Macrobdella decora OX=6405 PE=1 SV=1 |
| Q54727 | 2.64e-54 | 66 | 688 | 55 | 691 | Sialidase B OS=Streptococcus pneumoniae serotype 4 (strain ATCC BAA-334 / TIGR4) OX=170187 GN=nanB PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
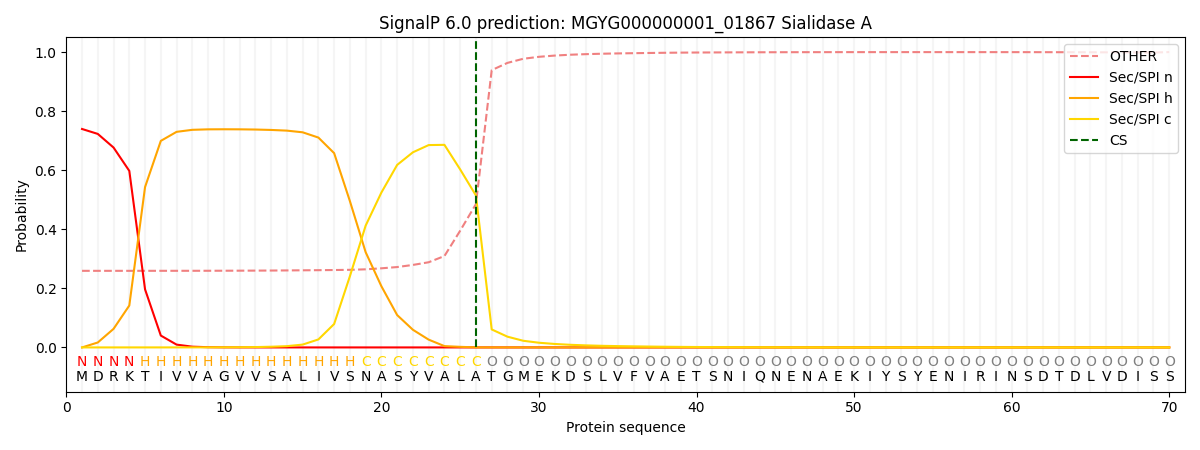
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.272626 | 0.724683 | 0.001488 | 0.000430 | 0.000363 | 0.000374 |