You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000054_00743
You are here: Home > Sequence: MGYG000000054_00743
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides acidifaciens | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides acidifaciens | |||||||||||
| CAZyme ID | MGYG000000054_00743 | |||||||||||
| CAZy Family | CE7 | |||||||||||
| CAZyme Description | Acetyl esterase Axe7A | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 38179; End: 39480 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE7 | 128 | 421 | 3.1e-83 | 0.9584664536741214 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3458 | Axe1 | 5.32e-40 | 136 | 428 | 21 | 316 | Cephalosporin-C deacetylase or related acetyl esterase [Secondary metabolites biosynthesis, transport and catabolism]. |
| pfam05448 | AXE1 | 2.32e-37 | 128 | 408 | 12 | 299 | Acetyl xylan esterase (AXE1). This family consists of several bacterial acetyl xylan esterase proteins. Acetyl xylan esterases are enzymes that hydrolyze the ester linkages of the acetyl groups in position 2 and/or 3 of the xylose moieties of natural acetylated xylan from hardwood. These enzymes are one of the accessory enzymes which are part of the xylanolytic system, together with xylanases, beta-xylosidases, alpha-arabinofuranosidases and methylglucuronidases; these are all required for the complete hydrolysis of xylan. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CBK67089.1 | 6.10e-312 | 1 | 431 | 1 | 432 |
| QUT79463.1 | 1.23e-311 | 1 | 431 | 1 | 432 |
| QDM10171.1 | 1.23e-311 | 1 | 431 | 1 | 432 |
| QDH53354.1 | 1.75e-311 | 1 | 431 | 1 | 432 |
| QRM99767.1 | 2.48e-311 | 1 | 431 | 1 | 432 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6AGQ_A | 1.71e-28 | 130 | 408 | 15 | 303 | Acetylxylan esterase from Paenibacillus sp. R4 [Paenibacillus sp. R4],6AGQ_B Acetyl xylan esterase from Paenibacillus sp. R4 [Paenibacillus sp. R4],6AGQ_C Acetyl xylan esterase from Paenibacillus sp. R4 [Paenibacillus sp. R4],6AGQ_D Acetyl xylan esterase from Paenibacillus sp. R4 [Paenibacillus sp. R4],6AGQ_E Acetyl xylan esterase from Paenibacillus sp. R4 [Paenibacillus sp. R4],6AGQ_F Acetyl xylan esterase from Paenibacillus sp. R4 [Paenibacillus sp. R4] |
| 5HFN_A | 9.65e-28 | 127 | 408 | 24 | 289 | Crystalstructure of a loop truncation variant of Thermotoga maritima Acetyl Esterase TM0077 (apo structure) at 2.75 Angstrom resolution [Thermotoga maritima MSB8],5HFN_B Crystal structure of a loop truncation variant of Thermotoga maritima Acetyl Esterase TM0077 (apo structure) at 2.75 Angstrom resolution [Thermotoga maritima MSB8],5HFN_C Crystal structure of a loop truncation variant of Thermotoga maritima Acetyl Esterase TM0077 (apo structure) at 2.75 Angstrom resolution [Thermotoga maritima MSB8],5HFN_D Crystal structure of a loop truncation variant of Thermotoga maritima Acetyl Esterase TM0077 (apo structure) at 2.75 Angstrom resolution [Thermotoga maritima MSB8],5HFN_E Crystal structure of a loop truncation variant of Thermotoga maritima Acetyl Esterase TM0077 (apo structure) at 2.75 Angstrom resolution [Thermotoga maritima MSB8],5HFN_F Crystal structure of a loop truncation variant of Thermotoga maritima Acetyl Esterase TM0077 (apo structure) at 2.75 Angstrom resolution [Thermotoga maritima MSB8] |
| 1ODS_A | 3.94e-27 | 130 | 408 | 15 | 298 | CephalosporinC deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_B Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_C Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_D Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_E Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_F Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_G Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis],1ODS_H Cephalosporin C deacetylase from Bacillus subtilis [Bacillus subtilis] |
| 1L7A_A | 3.94e-27 | 130 | 408 | 15 | 298 | structuralGenomics, crystal structure of Cephalosporin C deacetylase [Bacillus subtilis],1L7A_B structural Genomics, crystal structure of Cephalosporin C deacetylase [Bacillus subtilis] |
| 5GMA_A | 5.48e-27 | 127 | 408 | 24 | 315 | Crystalstructure of the P228A variant of Thermotoga maritima acetyl esterase [Thermotoga maritima MSB8],5GMA_B Crystal structure of the P228A variant of Thermotoga maritima acetyl esterase [Thermotoga maritima MSB8],5GMA_C Crystal structure of the P228A variant of Thermotoga maritima acetyl esterase [Thermotoga maritima MSB8],5GMA_D Crystal structure of the P228A variant of Thermotoga maritima acetyl esterase [Thermotoga maritima MSB8],5GMA_E Crystal structure of the P228A variant of Thermotoga maritima acetyl esterase [Thermotoga maritima MSB8],5GMA_F Crystal structure of the P228A variant of Thermotoga maritima acetyl esterase [Thermotoga maritima MSB8] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| D5EXI2 | 3.35e-95 | 35 | 413 | 46 | 425 | Acetyl esterase Axe7A OS=Prevotella ruminicola (strain ATCC 19189 / JCM 8958 / 23) OX=264731 GN=axe7A PE=1 SV=1 |
| P94388 | 1.57e-26 | 130 | 408 | 15 | 298 | Cephalosporin-C deacetylase OS=Bacillus subtilis (strain 168) OX=224308 GN=cah PE=1 SV=1 |
| Q9WXT2 | 4.61e-26 | 127 | 408 | 12 | 303 | Cephalosporin-C deacetylase OS=Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8) OX=243274 GN=axeA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
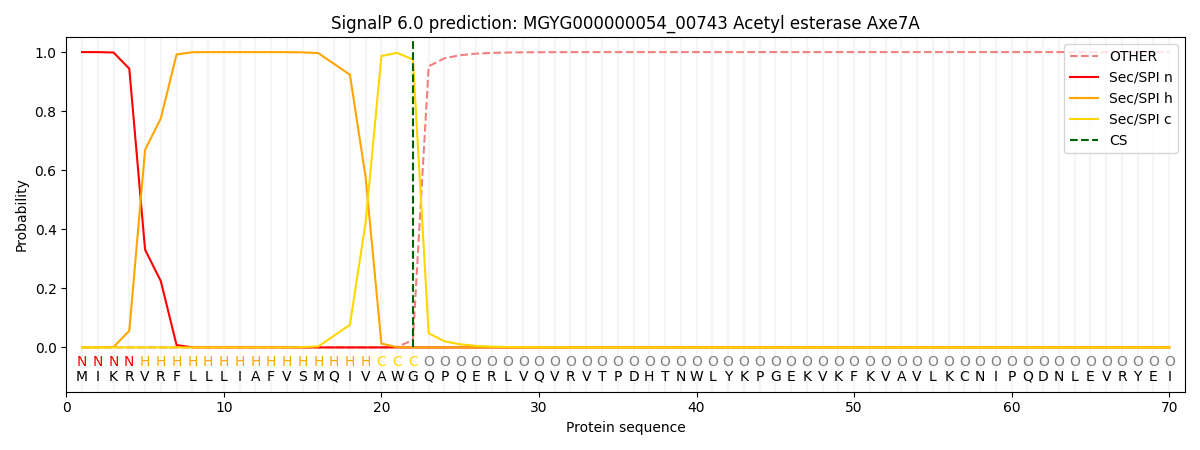
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000279 | 0.999048 | 0.000174 | 0.000169 | 0.000153 | 0.000140 |
