You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000055_00054
You are here: Home > Sequence: MGYG000000055_00054
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Muribaculum intestinale | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Muribaculaceae; Muribaculum; Muribaculum intestinale | |||||||||||
| CAZyme ID | MGYG000000055_00054 | |||||||||||
| CAZy Family | GH2 | |||||||||||
| CAZyme Description | Beta-galactosidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 66685; End: 70092 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH2 | 73 | 506 | 8.9e-74 | 0.4946808510638298 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3250 | LacZ | 3.39e-31 | 130 | 483 | 103 | 439 | Beta-galactosidase/beta-glucuronidase [Carbohydrate transport and metabolism]. |
| PRK10150 | PRK10150 | 2.89e-21 | 106 | 520 | 62 | 479 | beta-D-glucuronidase; Provisional |
| pfam07691 | PA14 | 3.79e-21 | 854 | 985 | 6 | 140 | PA14 domain. This domain forms an insert in bacterial beta-glucosidases and is found in other glycosidases, glycosyltransferases, proteases, amidases, yeast adhesins, and bacterial toxins, including anthrax protective antigen (PA). The domain also occurs in a Dictyostelium prespore-cell-inducing factor Psi and in fibrocystin, the mammalian protein whose mutation leads to polycystic kidney and hepatic disease. The crystal structure of PA shows that this domain (named PA14 after its location in the PA20 pro-peptide) has a beta-barrel structure. The PA14 domain sequence suggests a binding function, rather than a catalytic role. The PA14 domain distribution is compatible with carbohydrate binding. |
| PRK10340 | ebgA | 2.25e-17 | 115 | 482 | 115 | 470 | cryptic beta-D-galactosidase subunit alpha; Reviewed |
| smart00758 | PA14 | 2.82e-16 | 843 | 984 | 7 | 135 | domain in bacterial beta-glucosidases other glycosidases, glycosyltransferases, proteases, amidases, yeast adhesins, and bacterial toxins. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ANU64711.2 | 0.0 | 1 | 1135 | 1 | 1135 |
| QQR09994.1 | 0.0 | 1 | 1135 | 1 | 1135 |
| ASB36840.1 | 0.0 | 1 | 1135 | 1 | 1135 |
| QQA08720.1 | 0.0 | 29 | 1128 | 26 | 1127 |
| QMW85532.1 | 0.0 | 29 | 1128 | 26 | 1127 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6ED1_A | 4.12e-26 | 108 | 522 | 76 | 490 | ChainA, Glycosyl hydrolase family 2, sugar binding domain protein [Phocaeicola dorei],6ED1_B Chain B, Glycosyl hydrolase family 2, sugar binding domain protein [Phocaeicola dorei],6ED1_C Chain C, Glycosyl hydrolase family 2, sugar binding domain protein [Phocaeicola dorei],6ED1_D Chain D, Glycosyl hydrolase family 2, sugar binding domain protein [Phocaeicola dorei] |
| 4YPJ_A | 3.22e-22 | 109 | 486 | 70 | 441 | ChainA, Beta galactosidase [Niallia circulans],4YPJ_B Chain B, Beta galactosidase [Niallia circulans] |
| 6D7J_A | 1.31e-21 | 73 | 453 | 46 | 430 | TheCrystal Structure of Parabacteroides merdae Beta-Glucuronidase (GUS) with Glycerol in Active-Site [Parabacteroides merdae CL03T12C32],6D7J_B The Crystal Structure of Parabacteroides merdae Beta-Glucuronidase (GUS) with Glycerol in Active-Site [Parabacteroides merdae CL03T12C32],6D7J_C The Crystal Structure of Parabacteroides merdae Beta-Glucuronidase (GUS) with Glycerol in Active-Site [Parabacteroides merdae CL03T12C32],6D7J_D The Crystal Structure of Parabacteroides merdae Beta-Glucuronidase (GUS) with Glycerol in Active-Site [Parabacteroides merdae CL03T12C32],6DXU_A Crystal Structure of Parabacteroides merdae Beta-Glucuronidase (GUS) [Parabacteroides merdae ATCC 43184],6DXU_B Crystal Structure of Parabacteroides merdae Beta-Glucuronidase (GUS) [Parabacteroides merdae ATCC 43184],6DXU_C Crystal Structure of Parabacteroides merdae Beta-Glucuronidase (GUS) [Parabacteroides merdae ATCC 43184],6DXU_D Crystal Structure of Parabacteroides merdae Beta-Glucuronidase (GUS) [Parabacteroides merdae ATCC 43184] |
| 6B6L_A | 2.23e-19 | 88 | 485 | 48 | 435 | Thecrystal structure of glycosyl hydrolase family 2 (GH2) member from Bacteroides cellulosilyticus DSM 14838 [Bacteroides cellulosilyticus DSM 14838],6B6L_B The crystal structure of glycosyl hydrolase family 2 (GH2) member from Bacteroides cellulosilyticus DSM 14838 [Bacteroides cellulosilyticus DSM 14838] |
| 7RSK_A | 3.88e-19 | 88 | 485 | 48 | 435 | ChainA, Glycosyl hydrolase family 2, sugar binding domain protein [Bacteroides cellulosilyticus DSM 14838],7RSK_B Chain B, Glycosyl hydrolase family 2, sugar binding domain protein [Bacteroides cellulosilyticus DSM 14838] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| T2KM09 | 7.53e-22 | 109 | 489 | 106 | 459 | Putative beta-glucuronidase OS=Formosa agariphila (strain DSM 15362 / KCTC 12365 / LMG 23005 / KMM 3901 / M-2Alg 35-1) OX=1347342 GN=BN863_22050 PE=2 SV=2 |
| P26257 | 3.33e-18 | 130 | 482 | 75 | 411 | Beta-galactosidase OS=Thermoanaerobacterium thermosulfurigenes OX=33950 GN=lacZ PE=1 SV=1 |
| P77989 | 1.05e-17 | 105 | 453 | 52 | 388 | Beta-galactosidase OS=Thermoanaerobacter pseudethanolicus (strain ATCC 33223 / 39E) OX=340099 GN=lacZ PE=3 SV=2 |
| T2KN75 | 2.68e-16 | 105 | 448 | 83 | 433 | Beta-glucuronidase OS=Formosa agariphila (strain DSM 15362 / KCTC 12365 / LMG 23005 / KMM 3901 / M-2Alg 35-1) OX=1347342 GN=BN863_22060 PE=1 SV=1 |
| Q56307 | 2.50e-15 | 102 | 469 | 105 | 458 | Beta-galactosidase OS=Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8) OX=243274 GN=lacZ PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
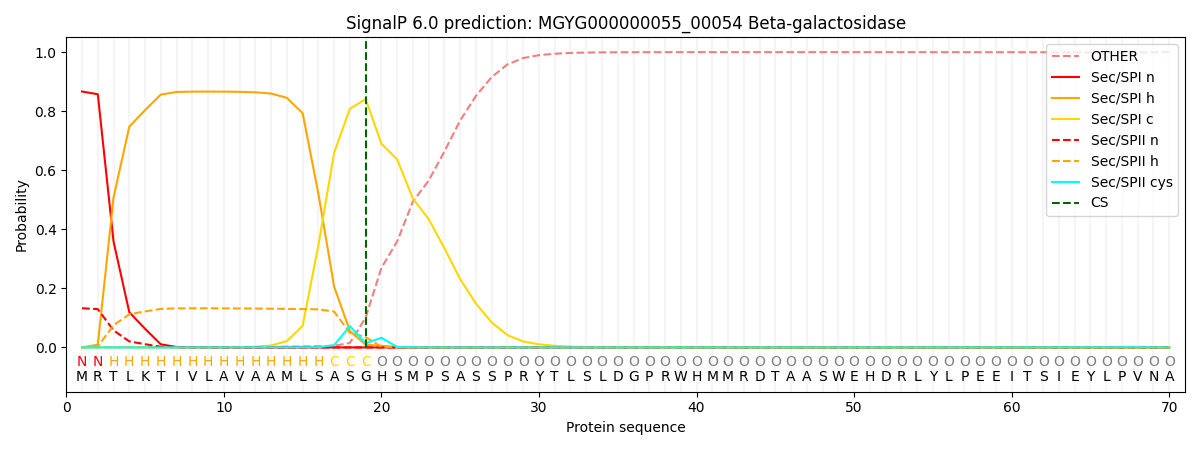
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.002309 | 0.858914 | 0.137844 | 0.000322 | 0.000303 | 0.000283 |
