You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000057_00886
You are here: Home > Sequence: MGYG000000057_00886
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides sp002491635 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides sp002491635 | |||||||||||
| CAZyme ID | MGYG000000057_00886 | |||||||||||
| CAZy Family | GH9 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 55518; End: 58052 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH9 | 406 | 778 | 8.8e-29 | 0.8253588516746412 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd02850 | E_set_Cellulase_N | 4.46e-21 | 302 | 389 | 1 | 86 | N-terminal Early set domain associated with the catalytic domain of cellulase. E or "early" set domains are associated with the catalytic domain of cellulases at the N-terminal end. Cellulases are O-glycosyl hydrolases (GHs) that hydrolyze beta 1-4 glucosidic bonds in cellulose. They are usually categorized into either exoglucanases, which sequentially release terminal sugar units from the cellulose chain, or endoglucanases, which also attack the chain internally. The N-terminal domain of cellulase may be related to the immunoglobulin and/or fibronectin type III superfamilies. These domains are associated with different types of catalytic domains at either the N-terminal or C-terminal end and may be involved in homodimeric/tetrameric/dodecameric interactions. Members of this family include members of the alpha amylase family, sialidase, galactose oxidase, cellulase, cellulose, hyaluronate lyase, chitobiase, and chitinase, among others. |
| pfam02927 | CelD_N | 2.21e-09 | 301 | 384 | 1 | 83 | Cellulase N-terminal ig-like domain. |
| pfam00759 | Glyco_hydro_9 | 2.66e-07 | 423 | 668 | 26 | 251 | Glycosyl hydrolase family 9. |
| PLN02171 | PLN02171 | 6.31e-06 | 581 | 785 | 172 | 410 | endoglucanase |
| PLN02613 | PLN02613 | 2.16e-04 | 578 | 716 | 165 | 323 | endoglucanase |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QKJ98593.1 | 5.32e-14 | 303 | 822 | 43 | 522 |
| AFN75471.1 | 8.53e-11 | 302 | 650 | 29 | 351 |
| AGH13480.1 | 1.04e-10 | 755 | 833 | 3 | 82 |
| AXR85444.1 | 1.92e-09 | 363 | 776 | 60 | 456 |
| SNV04832.1 | 2.80e-09 | 303 | 826 | 34 | 540 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3X17_A | 2.92e-10 | 303 | 805 | 18 | 522 | Crystalstructure of metagenome-derived glycoside hydrolase family 9 endoglucanase [uncultured bacterium],3X17_B Crystal structure of metagenome-derived glycoside hydrolase family 9 endoglucanase [uncultured bacterium] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
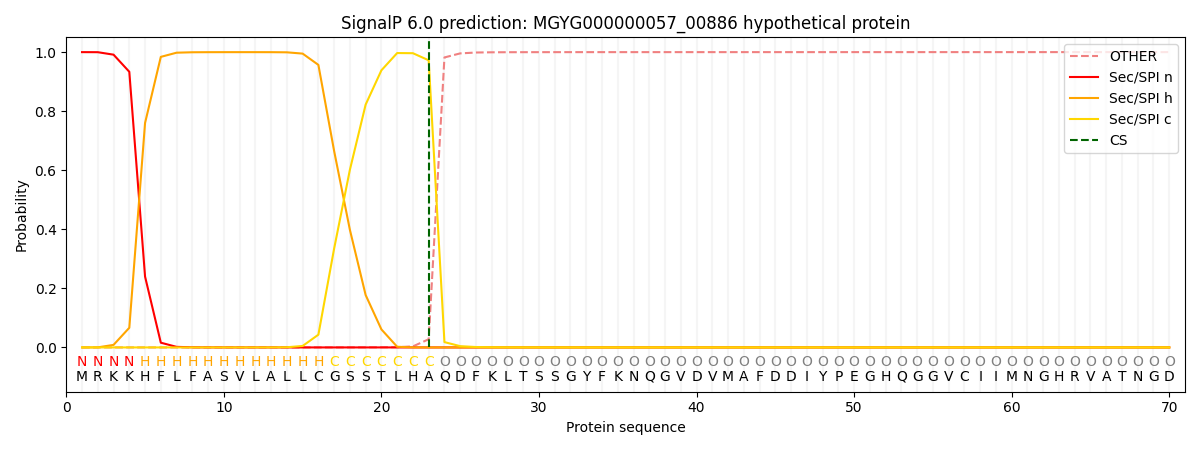
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000294 | 0.998533 | 0.000590 | 0.000188 | 0.000172 | 0.000158 |
