You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000064_00895
You are here: Home > Sequence: MGYG000000064_00895
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
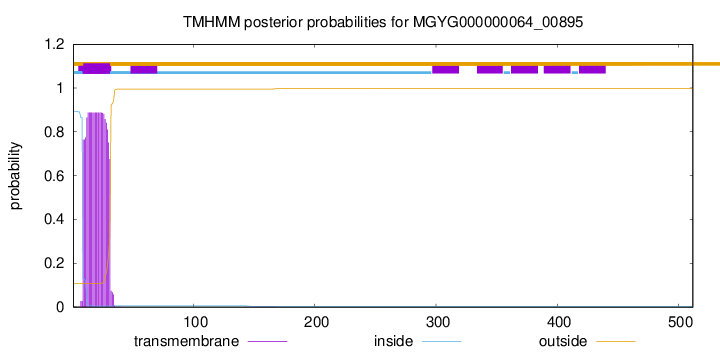
TMHMM annotations
Basic Information help
| Species | Clostridium baratii | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Clostridiales; Clostridiaceae; Clostridium; Clostridium baratii | |||||||||||
| CAZyme ID | MGYG000000064_00895 | |||||||||||
| CAZy Family | GH18 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 830805; End: 832343 Strand: + | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd06543 | GH18_PF-ChiA-like | 2.07e-96 | 60 | 371 | 1 | 294 | PF-ChiA is an uncharacterized chitinase found in the hyperthermophilic archaeon Pyrococcus furiosus with a glycosyl hydrolase family 18 (GH18) catalytic domain as well as a cellulose-binding domain. Members of this domain family are found not only in archaea but also in eukaryotes and prokaryotes. PF-ChiA exhibits hydrolytic activity toward both colloidal and crystalline (beta/alpha) chitins at high temperature. |
| pfam16403 | DUF5011 | 3.32e-19 | 391 | 461 | 3 | 71 | Domain of unknown function (DUF5011). This small family of proteins is functionally uncharacterized. This family is found in Bacteroides, Prevotella, and Parabateroides. Proteins in this family are around 230 amino acids in length. |
| cd12215 | ChiC_BD | 3.45e-10 | 470 | 510 | 1 | 42 | Chitin-binding domain of chitinase C. Chitin-binding domain of chitinase C (ChiC) of Streptomyces griseus and related proteins. Chitinase C is a family 19 chitinase, and consists of a N-terminal chitin binding domain and a C-terminal chitin-catalytic domain that effects degradation. Chitinases function in invertebrates in the degradation of old exoskeletons, in fungi to utilize chitin in cell walls, and in bacteria which use chitin as an energy source. ChiC contains the characteristic chitin-binding aromatic residues. |
| smart00495 | ChtBD3 | 5.48e-09 | 469 | 505 | 1 | 37 | Chitin-binding domain type 3. |
| cd00036 | ChtBD3 | 1.89e-06 | 472 | 510 | 2 | 40 | Chitin/cellulose binding domains of chitinase and related enzymes. This group contains proteins related to the cellulose-binding domain of Erwinia chrysanthemi endoglucanase Z (EGZ) and Serratia marcescens chitinase B (ChiB). Gram negative plant parasite Erwinia chrysanthemi produces a variety of depolymerizing enzymes to metabolize pectin and cellulose on the host plant. Cellulase EGZ has a modular structure, with an N-terminal catalytic domain linked to a C-terminal cellulose-binding domain (CBD). CBD mediates the secretion activity of EGZ. Chitinases allow certain bacteria to utilize chitin as a energy source. Typically, non-plant chitinases are of the glycosidase family 18. Bacillus circulans Glycosidase ChiA1 hydrolyzes chitin and is comprised of several domains: the C-terminal chitin binding domain, an N-terminal catalytic domain, and 2 fibronectin type III-like domains. Bacillus circulans WL-12 ChiA1 facilitates invasion of fungal cell walls. The ChiA1 chitin binding domain is required for the specific recognition of insoluble chitin. although topologically and structurally related, ChiA1 lacks the characteristic aromatic residues of Erwinia chrysanthemi endoglucanase Z (CBD(EGZ)). Streptomyces griseus Chitinase C is a family 19 chitinase, and consists of a N-terminal chitin binding domain and a C-terminal chitin-catalytic domain that effects degradation. ChiC contains the characteristic chitin-binding aromatic residues. Chitinases function in invertebrates in the degradation of old exoskeletons, in fungi to utilize chitin in cell walls, and in bacteria which use chitin as an energy source. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AIY82478.1 | 0.0 | 1 | 512 | 1 | 512 |
| AQM58882.1 | 0.0 | 1 | 512 | 1 | 512 |
| CDH91379.1 | 3.07e-235 | 37 | 512 | 64 | 537 |
| ACD22750.1 | 3.07e-235 | 37 | 512 | 64 | 537 |
| AIY82117.1 | 2.72e-231 | 37 | 512 | 64 | 537 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2DSK_A | 8.38e-23 | 58 | 351 | 8 | 263 | Crystalstructure of catalytic domain of hyperthermophilic chitinase from Pyrococcus furiosus [Pyrococcus furiosus],2DSK_B Crystal structure of catalytic domain of hyperthermophilic chitinase from Pyrococcus furiosus [Pyrococcus furiosus] |
| 3A4W_A | 5.25e-22 | 58 | 351 | 8 | 263 | Crystalstructures of catalytic site mutants of active domain 2 of thermostable chitinase from Pyrococcus furiosus complexed with chito-oligosaccharides [Pyrococcus furiosus],3A4W_B Crystal structures of catalytic site mutants of active domain 2 of thermostable chitinase from Pyrococcus furiosus complexed with chito-oligosaccharides [Pyrococcus furiosus] |
| 3A4X_A | 9.66e-22 | 58 | 351 | 8 | 263 | Crystalstructures of catalytic site mutants of active domain 2 of thermostable chitinase from Pyrococcus furiosus complexed with chito-oligosaccharides [Pyrococcus furiosus],3A4X_B Crystal structures of catalytic site mutants of active domain 2 of thermostable chitinase from Pyrococcus furiosus complexed with chito-oligosaccharides [Pyrococcus furiosus],3AFB_A Crystal structures of catalytic site mutants of active domain 2 of chitinase from Pyrococcus furiosus [Pyrococcus furiosus],3AFB_B Crystal structures of catalytic site mutants of active domain 2 of chitinase from Pyrococcus furiosus [Pyrococcus furiosus] |
| 4HMC_A | 1.17e-15 | 389 | 508 | 404 | 524 | Crystalstructure of cold-adapted chitinase from Moritella marina [Moritella marina],4HMD_A Crystal structure of cold-adapted chitinase from Moritella marina with a reaction intermediate - oxazolinium ion (NGO) [Moritella marina],4HME_A Crystal structure of cold-adapted chitinase from Moritella marina with a reaction product - NAG2 [Moritella marina] |
| 4MB3_A | 1.17e-15 | 389 | 508 | 404 | 524 | Crystalstructure of E153Q mutant of cold-adapted chitinase from Moritella marina [Moritella marina],4MB4_A Crystal structure of E153Q mutant of cold-adapted chitinase from Moritella complex with Nag4 [Moritella marina],4MB5_A Crystal structure of E153Q mutant of cold-adapted chitinase from Moritella complex with Nag5 [Moritella marina] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| O74199 | 3.82e-29 | 57 | 351 | 233 | 509 | Endochitinase 11 OS=Metarhizium anisopliae OX=5530 GN=chi11 PE=1 SV=1 |
| P13656 | 7.08e-29 | 57 | 351 | 584 | 863 | Probable bifunctional chitinase/lysozyme OS=Escherichia coli (strain K12) OX=83333 GN=chiA PE=1 SV=2 |
| P14529 | 3.03e-17 | 61 | 351 | 30 | 297 | Chitinase OS=Saccharopolyspora erythraea (strain ATCC 11635 / DSM 40517 / JCM 4748 / NBRC 13426 / NCIMB 8594 / NRRL 2338) OX=405948 GN=chiA2 PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
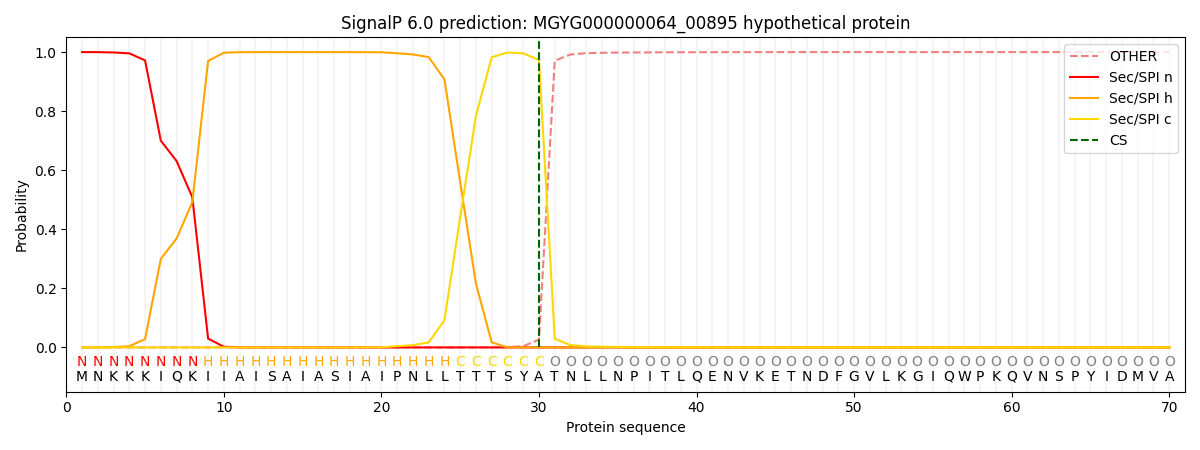
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000593 | 0.998439 | 0.000235 | 0.000264 | 0.000236 | 0.000201 |