You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000099_00991
You are here: Home > Sequence: MGYG000000099_00991
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
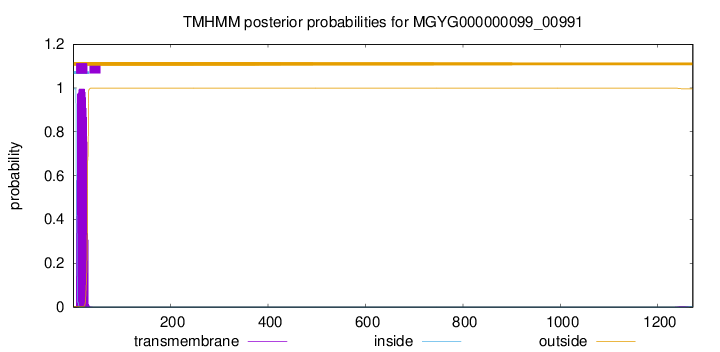
TMHMM annotations
Basic Information help
| Species | Flavonifractor plautii | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Oscillospiraceae; Flavonifractor; Flavonifractor plautii | |||||||||||
| CAZyme ID | MGYG000000099_00991 | |||||||||||
| CAZy Family | GH31 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 934997; End: 938815 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH31 | 247 | 697 | 1.4e-105 | 0.9976580796252927 |
| CBM35 | 850 | 979 | 2.9e-35 | 0.9915966386554622 |
| CBM61 | 1123 | 1268 | 2.8e-33 | 0.9716312056737588 |
| CBM35 | 989 | 1111 | 1.1e-31 | 0.9831932773109243 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG1501 | YicI | 7.43e-168 | 70 | 791 | 61 | 767 | Alpha-glucosidase, glycosyl hydrolase family GH31 [Carbohydrate transport and metabolism]. |
| pfam01055 | Glyco_hydro_31 | 8.59e-104 | 248 | 697 | 1 | 442 | Glycosyl hydrolases family 31. Glycosyl hydrolases are key enzymes of carbohydrate metabolism. Family 31 comprises of enzymes that are, or similar to, alpha- galactosidases. |
| cd06603 | GH31_GANC_GANAB_alpha | 2.58e-56 | 266 | 736 | 1 | 467 | neutral alpha-glucosidase C, neutral alpha-glucosidase AB. This subgroup includes the closely related glycosyl hydrolase family 31 (GH31) isozymes, neutral alpha-glucosidase C (GANC) and the alpha subunit of heterodimeric neutral alpha-glucosidase AB (GANAB). Initially distinguished on the basis of differences in electrophoretic mobility in starch gel, GANC and GANAB have been shown to have other differences, including those of substrate specificity. GANC and GANAB are key enzymes in glycogen metabolism that hydrolyze terminal, non-reducing 1,4-linked alpha-D-glucose residues from glycogen in the endoplasmic reticulum. The GANC/GANAB family includes the alpha-glucosidase II (ModA) from Dictyostelium discoideum as well as the alpha-glucosidase II (GLS2, or ROT2 - Reversal of TOR2 lethality protein 2) from Saccharomyces cerevisiae. |
| cd06589 | GH31 | 2.59e-53 | 266 | 587 | 1 | 265 | glycosyl hydrolase family 31 (GH31). GH31 enzymes occur in prokaryotes, eukaryotes, and archaea with a wide range of hydrolytic activities, including alpha-glucosidase (glucoamylase and sucrase-isomaltase), alpha-xylosidase, 6-alpha-glucosyltransferase, 3-alpha-isomaltosyltransferase and alpha-1,4-glucan lyase. All GH31 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. In most cases, the pyranose moiety recognized in subsite -1 of the substrate binding site is an alpha-D-glucose, though some GH31 family members show a preference for alpha-D-xylose. Several GH31 enzymes can accommodate both glucose and xylose and different levels of discrimination between the two have been observed. Most characterized GH31 enzymes are alpha-glucosidases. In mammals, GH31 members with alpha-glucosidase activity are implicated in at least three distinct biological processes. The lysosomal acid alpha-glucosidase (GAA) is essential for glycogen degradation and a deficiency or malfunction of this enzyme causes glycogen storage disease II, also known as Pompe disease. In the endoplasmic reticulum, alpha-glucosidase II catalyzes the second step in the N-linked oligosaccharide processing pathway that constitutes part of the quality control system for glycoprotein folding and maturation. The intestinal enzymes sucrase-isomaltase (SI) and maltase-glucoamylase (MGAM) play key roles in the final stage of carbohydrate digestion, making alpha-glucosidase inhibitors useful in the treatment of type 2 diabetes. GH31 alpha-glycosidases are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| cd06598 | GH31_transferase_CtsZ | 1.32e-52 | 266 | 599 | 1 | 328 | CtsZ (cyclic tetrasaccharide-synthesizing enzyme Z)-like. CtsZ is a bacterial 6-alpha-glucosyltransferase, first identified in Arthrobacter globiformis, that produces cyclic tetrasaccharides together with a closely related enzyme CtsY. CtsZ and CtsY both have a glycosyl hydrolase family 31 (GH31) catalytic domain; CtsY belongs to a different subfamily. All GH31 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ANU41627.1 | 0.0 | 1 | 1272 | 1 | 1272 |
| QQR05509.1 | 0.0 | 1 | 1272 | 1 | 1272 |
| QIA31697.1 | 0.0 | 1 | 1272 | 1 | 1272 |
| SET57319.1 | 0.0 | 28 | 1269 | 30 | 1273 |
| QEY34937.1 | 0.0 | 28 | 1272 | 35 | 1282 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5X7O_A | 0.0 | 35 | 1269 | 20 | 1259 | Crystalstructure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase [Paenibacillus sp. 598K],5X7O_B Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase [Paenibacillus sp. 598K],5X7P_A Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase complexed with acarbose [Paenibacillus sp. 598K],5X7P_B Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase complexed with acarbose [Paenibacillus sp. 598K],5X7Q_A Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase complexed with maltohexaose [Paenibacillus sp. 598K],5X7Q_B Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase complexed with maltohexaose [Paenibacillus sp. 598K],5X7R_A Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase complexed with isomaltohexaose [Paenibacillus sp. 598K],5X7R_B Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase complexed with isomaltohexaose [Paenibacillus sp. 598K],5X7S_A Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase, terbium derivative [Paenibacillus sp. 598K],5X7S_B Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase, terbium derivative [Paenibacillus sp. 598K] |
| 6JR6_A | 1.61e-70 | 35 | 761 | 30 | 753 | Flavobacteriumjohnsoniae GH31 dextranase, FjDex31A [Flavobacterium johnsoniae UW101],6JR6_B Flavobacterium johnsoniae GH31 dextranase, FjDex31A [Flavobacterium johnsoniae UW101],6JR6_C Flavobacterium johnsoniae GH31 dextranase, FjDex31A [Flavobacterium johnsoniae UW101],6JR6_D Flavobacterium johnsoniae GH31 dextranase, FjDex31A [Flavobacterium johnsoniae UW101],6JR7_A Flavobacterium johnsoniae GH31 dextranase, FjDex31A, complexed with glucose [Flavobacterium johnsoniae UW101],6JR7_B Flavobacterium johnsoniae GH31 dextranase, FjDex31A, complexed with glucose [Flavobacterium johnsoniae UW101],6JR7_C Flavobacterium johnsoniae GH31 dextranase, FjDex31A, complexed with glucose [Flavobacterium johnsoniae UW101],6JR7_D Flavobacterium johnsoniae GH31 dextranase, FjDex31A, complexed with glucose [Flavobacterium johnsoniae UW101] |
| 6JR8_A | 3.96e-70 | 35 | 761 | 30 | 753 | Flavobacteriumjohnsoniae GH31 dextranase, FjDex31A, mutant D412A complexed with isomaltotriose [Flavobacterium johnsoniae UW101],6JR8_B Flavobacterium johnsoniae GH31 dextranase, FjDex31A, mutant D412A complexed with isomaltotriose [Flavobacterium johnsoniae UW101],6JR8_C Flavobacterium johnsoniae GH31 dextranase, FjDex31A, mutant D412A complexed with isomaltotriose [Flavobacterium johnsoniae UW101],6JR8_D Flavobacterium johnsoniae GH31 dextranase, FjDex31A, mutant D412A complexed with isomaltotriose [Flavobacterium johnsoniae UW101] |
| 5F7U_A | 2.44e-63 | 104 | 979 | 180 | 1061 | Cycloalternan-formingenzyme from Listeria monocytogenes in complex with pentasaccharide substrate [Listeria monocytogenes EGD-e] |
| 5I0D_A | 9.54e-60 | 104 | 979 | 180 | 1061 | Cycloalternan-formingenzyme from Listeria monocytogenes in complex with cycloalternan [Listeria monocytogenes EGD-e],5I0D_B Cycloalternan-forming enzyme from Listeria monocytogenes in complex with cycloalternan [Listeria monocytogenes EGD-e] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q9P999 | 9.52e-60 | 149 | 750 | 100 | 674 | Alpha-xylosidase OS=Saccharolobus solfataricus (strain ATCC 35092 / DSM 1617 / JCM 11322 / P2) OX=273057 GN=xylS PE=1 SV=1 |
| Q9F234 | 3.31e-56 | 245 | 792 | 230 | 769 | Alpha-glucosidase 2 OS=Bacillus thermoamyloliquefaciens OX=1425 PE=3 SV=1 |
| A7LXT0 | 7.45e-48 | 243 | 744 | 372 | 889 | Alpha-xylosidase BoGH31A OS=Bacteroides ovatus (strain ATCC 8483 / DSM 1896 / JCM 5824 / BCRC 10623 / CCUG 4943 / NCTC 11153) OX=411476 GN=BACOVA_02646 PE=1 SV=1 |
| Q9FN05 | 1.29e-40 | 245 | 744 | 332 | 828 | Probable glucan 1,3-alpha-glucosidase OS=Arabidopsis thaliana OX=3702 GN=PSL5 PE=1 SV=1 |
| B9F676 | 2.75e-39 | 247 | 744 | 333 | 826 | Probable glucan 1,3-alpha-glucosidase OS=Oryza sativa subsp. japonica OX=39947 GN=Os03g0216600 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP

| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000279 | 0.999052 | 0.000153 | 0.000192 | 0.000148 | 0.000138 |