You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000110_01866
You are here: Home > Sequence: MGYG000000110_01866
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Kluyvera sp902363335 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Proteobacteria; Gammaproteobacteria; Enterobacterales; Enterobacteriaceae; Kluyvera; Kluyvera sp902363335 | |||||||||||
| CAZyme ID | MGYG000000110_01866 | |||||||||||
| CAZy Family | GH24 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 615430; End: 615945 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH24 | 30 | 161 | 4.5e-47 | 0.9854014598540146 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd16901 | lyz_P1 | 5.52e-72 | 29 | 166 | 2 | 140 | P1 lysozyme Lyz-like proteins. Enterobacteria phage P1 lysozyme Lyz is secreted to the Escherichia coli periplasm where it is membrane bound and inactive. Activation involves the release from the membrane, an intramolecular thiol-disulfide isomerization and extensive structural rearrangement of the N-terminal region. The dsDNA phages of eubacteria use endolysins or muralytic enzymes in conjunction with hollin, a small membrane protein, to degrade the peptidoglycan found in bacterial cell walls. Similarly, bacteria produce autolysins to facilitate the biosynthesis of its cell wall heteropolymer peptidoglycan and cell division. Endolysins and autolysins are found in viruses and bacteria, respectively. Both endolysin and autolysin enzymes cleave the glycosidic beta 1,4-bonds between the N-acetylmuramic acid and the N-acetylglucosamine of the peptidoglycan. |
| cd00737 | lyz_endolysin_autolysin | 5.83e-54 | 33 | 163 | 1 | 133 | endolysin and autolysin. The dsDNA phages of eubacteria use endolysins or muralytic enzymes in conjunction with hollin, a small membrane protein, to degrade the peptidoglycan found in bacterial cell walls. Similarly, bacteria produce autolysins to facilitate the biosynthesis of its cell wall heteropolymer peptidoglycan and cell division. Endolysins and autolysins are found in viruses and bacteria, respectively. Both endolysin and autolysin enzymes cleave the glycosidic beta 1,4-bonds between the N-acetylmuramic acid and the N-acetylglucosamine of the peptidoglycan. |
| COG3772 | RrrD | 2.29e-49 | 29 | 169 | 7 | 151 | Phage-related lysozyme (muramidase), GH24 family [Cell wall/membrane/envelope biogenesis]. |
| cd16900 | endolysin_R21-like | 1.58e-41 | 12 | 166 | 1 | 142 | endolysin R21-like proteins. Unlike T4 E phage lysozyme, the endolysin R21 from Enterobacteria phage P21 has an N-terminal SAR (signal-arrest-release) domain that anchors the endolysin to the membrane in an inactive form, which act to prevent premature lysis of the infected bacterium. The dsDNA phages of eubacteria use endolysins or muralytic enzymes in conjunction with hollin, a small membrane protein, to degrade the peptidoglycan found in bacterial cell walls. Similarly, bacteria produce autolysins to facilitate the biosynthesis of its cell wall heteropolymer peptidoglycan and cell division. Endolysins and autolysins are found in viruses and bacteria, respectively. Both endolysin and autolysin enzymes cleave the glycosidic beta 1,4-bonds between the N-acetylmuramic acid and the N-acetylglucosamine of the peptidoglycan. |
| pfam00959 | Phage_lysozyme | 6.59e-21 | 54 | 159 | 1 | 107 | Phage lysozyme. This family includes lambda phage lysozyme and E. coli endolysin. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AXG20064.1 | 7.22e-114 | 1 | 170 | 1 | 170 |
| QAZ83361.1 | 7.22e-114 | 1 | 170 | 1 | 170 |
| APJ73221.1 | 7.22e-114 | 1 | 170 | 1 | 170 |
| QJF99062.1 | 7.22e-114 | 1 | 170 | 1 | 170 |
| QAZ84903.1 | 7.22e-114 | 1 | 170 | 1 | 170 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1XJT_A | 3.26e-28 | 12 | 168 | 10 | 181 | ChainA, Lysozyme [Punavirus P1] |
| 1XJU_A | 1.25e-27 | 28 | 168 | 1 | 153 | ChainA, Lysozyme [Punavirus P1],1XJU_B Chain B, Lysozyme [Punavirus P1] |
| 3HDE_A | 5.34e-20 | 1 | 168 | 1 | 163 | ChainA, Lysozyme [Enterobacteria phage P21],3HDE_B Chain B, Lysozyme [Enterobacteria phage P21],3HDE_C Chain C, Lysozyme [Enterobacteria phage P21],3HDE_D Chain D, Lysozyme [Enterobacteria phage P21] |
| 4ZPU_A | 9.45e-20 | 1 | 168 | 1 | 163 | Thestructure of DLP12 endolysin exhibits likely active and inactive conformations. [Escherichia coli K-12],4ZPU_B The structure of DLP12 endolysin exhibits likely active and inactive conformations. [Escherichia coli K-12],4ZPU_C The structure of DLP12 endolysin exhibits likely active and inactive conformations. [Escherichia coli K-12],4ZPU_D The structure of DLP12 endolysin exhibits likely active and inactive conformations. [Escherichia coli K-12] |
| 3HDF_A | 8.96e-19 | 41 | 168 | 10 | 138 | ChainA, Lysozyme [Enterobacteria phage P21],3HDF_B Chain B, Lysozyme [Enterobacteria phage P21] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q37875 | 1.94e-28 | 12 | 168 | 10 | 181 | SAR-endolysin OS=Escherichia phage P1 OX=2886926 GN=17 PE=1 SV=1 |
| Q6XQ98 | 6.58e-24 | 7 | 167 | 4 | 160 | SAR-endolysin OS=Escherichia phage T1 OX=1921008 GN=12 PE=3 SV=1 |
| P51728 | 5.61e-21 | 21 | 166 | 27 | 184 | SAR-endolysin OS=Haemophilus phage HP1 (strain HP1c1) OX=1289570 GN=lys PE=3 SV=1 |
| Q9T1T5 | 8.44e-21 | 27 | 163 | 1 | 141 | Endolysin OS=Acyrthosiphon pisum secondary endosymbiont phage 1 OX=2682836 GN=13 PE=3 SV=1 |
| P07540 | 8.54e-21 | 27 | 163 | 1 | 140 | Endolysin OS=Bacillus phage PZA OX=10757 GN=15 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
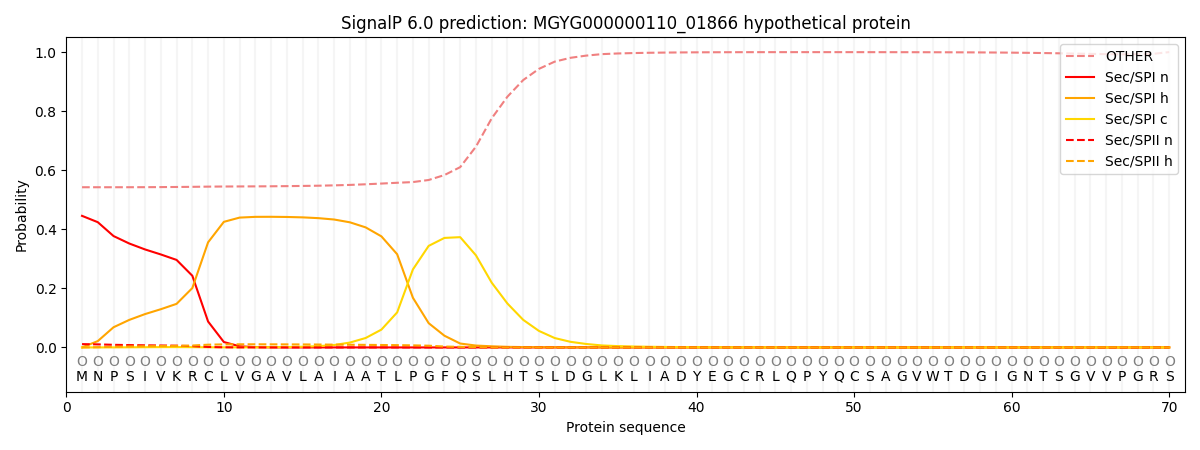
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.563177 | 0.418811 | 0.013583 | 0.001265 | 0.000789 | 0.002365 |
