You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000147_01953
You are here: Home > Sequence: MGYG000000147_01953
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
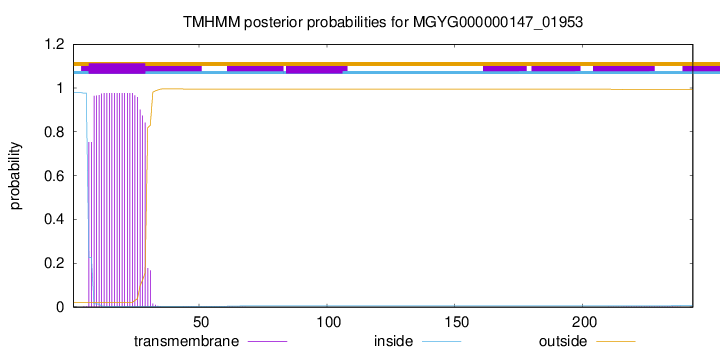
TMHMM annotations
Basic Information help
| Species | Bacillus paralicheniformis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Bacillales; Bacillaceae; Bacillus; Bacillus paralicheniformis | |||||||||||
| CAZyme ID | MGYG000000147_01953 | |||||||||||
| CAZy Family | GH16 | |||||||||||
| CAZyme Description | Beta-glucanase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 164929; End: 165660 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH16 | 37 | 239 | 4.6e-92 | 0.9951456310679612 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd02175 | GH16_lichenase | 7.27e-124 | 35 | 242 | 1 | 212 | lichenase, member of glycosyl hydrolase family 16. Lichenase, also known as 1,3-1,4-beta-glucanase, is a member of glycosyl hydrolase family 16, that specifically cleaves 1,4-beta-D-glucosidic bonds in mixed-linked beta glucans that also contain 1,3-beta-D-glucosidic linkages. Natural substrates of beta-glucanase are beta-glucans from grain endosperm cell walls or lichenan from the Islandic moss, Cetraria islandica. This protein is found not only in bacteria but also in anaerobic fungi. This domain includes two seven-stranded antiparallel beta-sheets that are adjacent to one another forming a compact, jellyroll beta-sandwich structure. |
| COG2273 | BglS | 1.09e-64 | 21 | 242 | 33 | 267 | Beta-glucanase, GH16 family [Carbohydrate transport and metabolism]. |
| cd00413 | Glyco_hydrolase_16 | 8.80e-54 | 37 | 241 | 1 | 210 | glycosyl hydrolase family 16. The O-Glycosyl hydrolases are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A glycosyl hydrolase classification system based on sequence similarity has led to the definition of more than 95 different families inlcuding glycosyl hydrolase family 16. Family 16 includes lichenase, xyloglucan endotransglycosylase (XET), beta-agarase, kappa-carrageenase, endo-beta-1,3-glucanase, endo-beta-1,3-1,4-glucanase, and endo-beta-galactosidase, all of which have a conserved jelly roll fold with a deep active site channel harboring the catalytic residues. |
| pfam00722 | Glyco_hydro_16 | 2.29e-52 | 63 | 239 | 1 | 168 | Glycosyl hydrolases family 16. |
| cd02183 | GH16_fungal_CRH1_transglycosylase | 2.83e-24 | 101 | 235 | 47 | 194 | glycosylphosphatidylinositol-glucanosyltransferase. Group of fungal GH16 members related to Saccharomyces cerevisiae Crh1p. Chr1p and Crh2p are transglycosylases that are required for the linkage of chitin to beta(1-3)glucose branches of beta(1-6)glucan, an important step in the assembly of new cell wall. Both have been shown to be glycosylphosphatidylinositol (GPI)-anchored. A third homologous protein, Crr1p, functions in the formation of the spore wall. They belongs to the family 16 of glycosyl hydrolases that includes lichenase, xyloglucan endotransglycosylase (XET), beta-agarase, kappa-carrageenase, endo-beta-1,3-glucanase, endo-beta-1,3-1,4-glucanase, and endo-beta-galactosidase, all of which have a conserved jelly roll fold with a deep active site channel harboring the catalytic residues. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ARA86799.1 | 6.96e-186 | 1 | 243 | 1 | 243 |
| AJO19490.1 | 6.96e-186 | 1 | 243 | 1 | 243 |
| QSF99896.1 | 1.99e-185 | 1 | 243 | 1 | 243 |
| VEB20262.1 | 1.99e-185 | 1 | 243 | 1 | 243 |
| QFY37755.1 | 4.03e-185 | 1 | 243 | 1 | 243 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3O5S_A | 9.75e-159 | 27 | 243 | 22 | 238 | CrystalStructure of the endo-beta-1,3-1,4 glucanase from Bacillus subtilis (strain 168) [Bacillus subtilis] |
| 1GBG_A | 3.22e-156 | 30 | 243 | 1 | 214 | BacillusLicheniformis Beta-Glucanase [Bacillus licheniformis] |
| 1BYH_A | 8.52e-136 | 30 | 241 | 1 | 212 | MOLECULARAND ACTIVE-SITE STRUCTURE OF A BACILLUS (1-3,1-4)-BETA-GLUCANASE [synthetic construct],1GLH_A Cation Binding To A Bacillus (1,3-1,4)-Beta-Glucanase. Geometry, Affinity And Effect On Protein Stability [Paenibacillus macerans],2AYH_A Crystal And Molecular Structure At 1.6 Angstroms Resolution Of The Hybrid Bacillus Endo-1,3-1,4-Beta-D-Glucan 4- Glucanohydrolase H(A16-M) [hybrid] |
| 1U0A_A | 6.99e-135 | 30 | 241 | 1 | 212 | ChainA, Beta-glucanase [Paenibacillus macerans],1U0A_B Chain B, Beta-glucanase [Paenibacillus macerans],1U0A_C Chain C, Beta-glucanase [Paenibacillus macerans],1U0A_D Chain D, Beta-glucanase [Paenibacillus macerans] |
| 3D6E_A | 3.45e-132 | 30 | 243 | 1 | 201 | Crystalstructure of the engineered 1,3-1,4-beta-glucanase protein from Bacillus licheniformis [Bacillus licheniformis],3D6E_B Crystal structure of the engineered 1,3-1,4-beta-glucanase protein from Bacillus licheniformis [Bacillus licheniformis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P04957 | 2.38e-171 | 1 | 243 | 1 | 242 | Beta-glucanase OS=Bacillus subtilis (strain 168) OX=224308 GN=bglS PE=1 SV=2 |
| P07980 | 1.32e-165 | 5 | 243 | 1 | 239 | Beta-glucanase OS=Bacillus amyloliquefaciens OX=1390 GN=bglA PE=3 SV=1 |
| P27051 | 2.08e-163 | 1 | 243 | 1 | 243 | Beta-glucanase OS=Bacillus licheniformis OX=1402 GN=bg1 PE=1 SV=1 |
| P23904 | 3.74e-128 | 26 | 241 | 20 | 235 | Beta-glucanase OS=Paenibacillus macerans OX=44252 PE=1 SV=2 |
| P45797 | 7.81e-128 | 11 | 241 | 10 | 236 | Beta-glucanase OS=Paenibacillus polymyxa OX=1406 GN=gluB PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
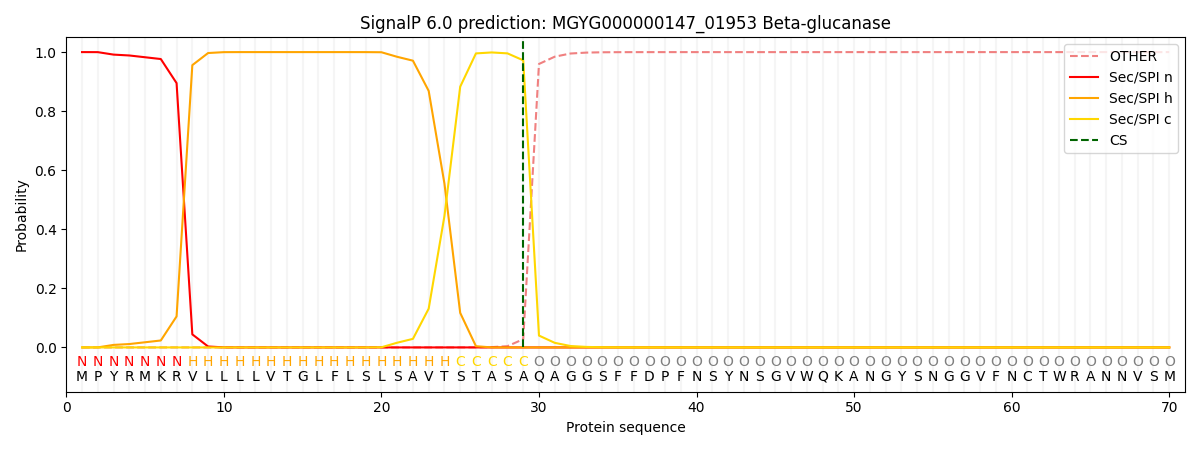
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000208 | 0.999185 | 0.000145 | 0.000169 | 0.000140 | 0.000128 |