You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000160_00259
You are here: Home > Sequence: MGYG000000160_00259
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
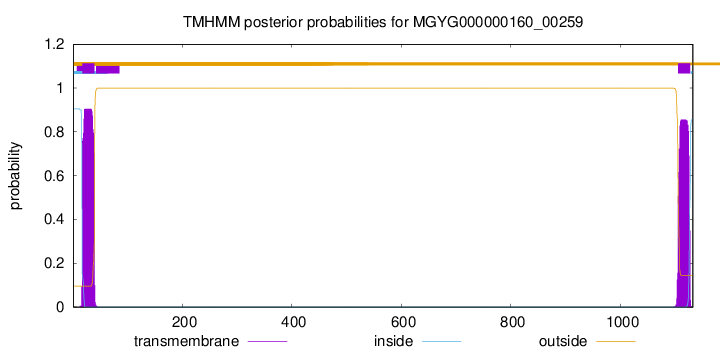
TMHMM annotations
Basic Information help
| Species | Collinsella sp003479805 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Actinobacteriota; Coriobacteriia; Coriobacteriales; Coriobacteriaceae; Collinsella; Collinsella sp003479805 | |||||||||||
| CAZyme ID | MGYG000000160_00259 | |||||||||||
| CAZy Family | GH51 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 318914; End: 322315 Strand: + | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3534 | AbfA | 9.02e-15 | 328 | 506 | 4 | 174 | Alpha-L-arabinofuranosidase [Carbohydrate transport and metabolism]. |
| pfam13385 | Laminin_G_3 | 2.71e-14 | 107 | 250 | 7 | 151 | Concanavalin A-like lectin/glucanases superfamily. This domain belongs to the Concanavalin A-like lectin/glucanases superfamily. |
| TIGR02243 | TIGR02243 | 1.32e-06 | 529 | 648 | 331 | 442 | putative baseplate assembly protein. This family consists of a large, conserved hypothetical protein in phage tail-like regions of at least six bacterial genomes: Gloeobacter violaceus PCC 7421, Geobacter sulfurreducens PCA, Streptomyces coelicolor A3(2), Streptomyces avermitilis MA-4680, Mesorhizobium loti, and Myxococcus xanthus. The C-terminal region is identified by the broader model pfam04865 as related to baseplate protein J from phage P2, but that relationship is not observed directly. [Mobile and extrachromosomal element functions, Prophage functions] |
| smart00159 | PTX | 9.39e-06 | 117 | 253 | 31 | 174 | Pentraxin / C-reactive protein / pentaxin family. This family form a doscoid pentameric structure. Human serum amyloid P demonstrates calcium-mediated ligand-binding. |
| pfam09479 | Flg_new | 2.41e-05 | 1008 | 1069 | 1 | 65 | Listeria-Bacteroides repeat domain (List_Bact_rpt). This model describes a conserved core region of about 43 residues, which occurs in at least two families of tandem repeats. These include 78-residue repeats which occur from 2 to 15 times in some proteins of Bacteroides forsythus ATCC 43037, and 70-residue repeats found in families of internalins of Listeria species. Single copies are found in proteins of Fibrobacter succinogenes, Geobacter sulfurreducens, and a few other bacteria. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BBA56144.1 | 6.52e-211 | 43 | 986 | 29 | 972 |
| AXM90856.1 | 1.47e-210 | 43 | 986 | 46 | 989 |
| BBA48000.1 | 2.55e-210 | 43 | 986 | 29 | 972 |
| ALE10461.1 | 3.59e-210 | 43 | 986 | 29 | 972 |
| ADO52227.1 | 8.10e-210 | 43 | 986 | 46 | 989 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4ATW_A | 1.61e-10 | 330 | 505 | 5 | 172 | Thecrystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_B The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_C The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_D The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_E The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8],4ATW_F The crystal structure of Arabinofuranosidase [Thermotoga maritima MSB8] |
| 3S2C_A | 1.62e-10 | 330 | 505 | 5 | 172 | Structureof the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_B Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_C Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_D Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_E Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_F Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_G Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_H Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_I Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_J Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_K Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1],3S2C_L Structure of the thermostable GH51 alpha-L-arabinofuranosidase from Thermotoga petrophila RKU-1 [Thermotoga petrophila RKU-1] |
| 3UG3_A | 1.70e-10 | 330 | 505 | 25 | 192 | Crystalstructure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_B Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_C Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_D Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_E Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG3_F Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima ligand free form [Thermotoga maritima],3UG4_A Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_B Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_C Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_D Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_E Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG4_F Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima arabinose complex [Thermotoga maritima],3UG5_A Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_B Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_C Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_D Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_E Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima],3UG5_F Crystal structure of alpha-L-arabinofuranosidase from Thermotoga maritima xylose complex [Thermotoga maritima] |
| 6ZPS_AAA | 1.97e-07 | 361 | 505 | 204 | 351 | ChainAAA, MgGH51 [Meripilus giganteus],6ZPV_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZPW_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZPX_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZPY_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZPZ_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZQ0_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZQ1_AAA Chain AAA, MgGH51 [Meripilus giganteus] |
| 2VRK_A | 2.01e-06 | 366 | 506 | 52 | 177 | Structureof a seleno-methionyl derivative of wild type arabinofuranosidase from Thermobacillus xylanilyticus [Thermobacillus xylanilyticus],2VRK_B Structure of a seleno-methionyl derivative of wild type arabinofuranosidase from Thermobacillus xylanilyticus [Thermobacillus xylanilyticus],2VRK_C Structure of a seleno-methionyl derivative of wild type arabinofuranosidase from Thermobacillus xylanilyticus [Thermobacillus xylanilyticus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P53627 | 1.01e-10 | 356 | 505 | 41 | 175 | Intracellular exo-alpha-(1->5)-L-arabinofuranosidase OS=Streptomyces lividans OX=1916 GN=abfA PE=1 SV=1 |
| E7CY70 | 2.22e-09 | 356 | 505 | 53 | 186 | Exo-alpha-(1->6)-L-arabinofuranosidase OS=Bifidobacterium longum OX=216816 GN=afuB PE=1 SV=1 |
| P94552 | 3.34e-08 | 361 | 506 | 47 | 173 | Intracellular exo-alpha-L-arabinofuranosidase 2 OS=Bacillus subtilis (strain 168) OX=224308 GN=abf2 PE=1 SV=2 |
| P94531 | 1.02e-07 | 360 | 505 | 45 | 173 | Intracellular exo-alpha-(1->5)-L-arabinofuranosidase 1 OS=Bacillus subtilis (strain 168) OX=224308 GN=abfA PE=1 SV=2 |
| U6A629 | 6.27e-07 | 361 | 507 | 211 | 358 | Alpha-L-arabinofuranosidase A OS=Penicillium canescens OX=5083 GN=abfA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
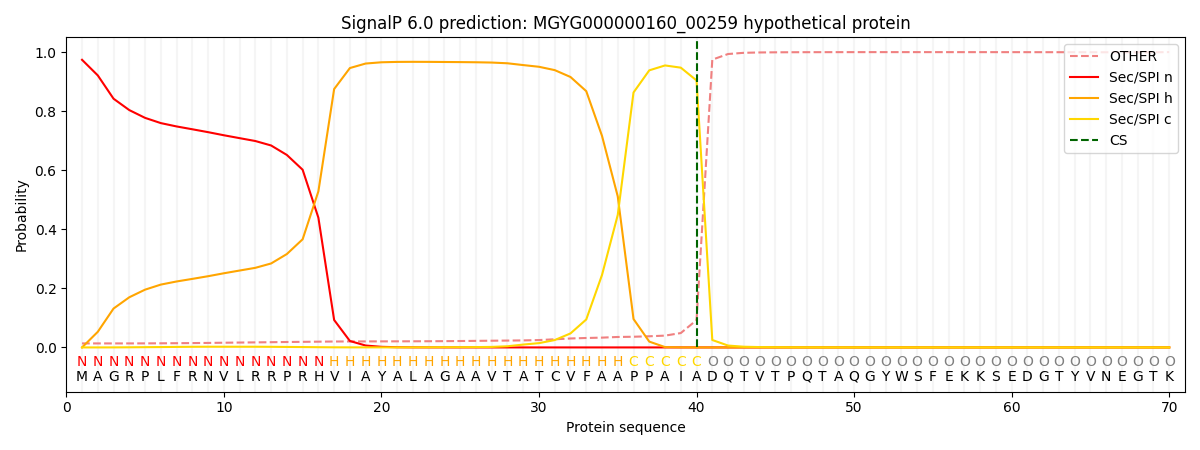
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.015118 | 0.970455 | 0.008959 | 0.004885 | 0.000305 | 0.000232 |