You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000211_01988
You are here: Home > Sequence: MGYG000000211_01988
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides sp900556215 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides sp900556215 | |||||||||||
| CAZyme ID | MGYG000000211_01988 | |||||||||||
| CAZy Family | GH2 | |||||||||||
| CAZyme Description | Beta-galactosidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 311575; End: 314457 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH2 | 21 | 665 | 3.6e-68 | 0.6422872340425532 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| PRK10150 | PRK10150 | 2.21e-21 | 26 | 348 | 14 | 296 | beta-D-glucuronidase; Provisional |
| COG3250 | LacZ | 3.04e-18 | 27 | 482 | 15 | 433 | Beta-galactosidase/beta-glucuronidase [Carbohydrate transport and metabolism]. |
| PRK10340 | ebgA | 5.78e-13 | 23 | 384 | 40 | 373 | cryptic beta-D-galactosidase subunit alpha; Reviewed |
| pfam02837 | Glyco_hydro_2_N | 6.34e-13 | 24 | 220 | 1 | 169 | Glycosyl hydrolases family 2, sugar binding domain. This family contains beta-galactosidase, beta-mannosidase and beta-glucuronidase activities and has a jelly-roll fold. The domain binds the sugar moiety during the sugar-hydrolysis reaction. |
| pfam00703 | Glyco_hydro_2 | 6.99e-10 | 222 | 328 | 1 | 106 | Glycosyl hydrolases family 2. This family contains beta-galactosidase, beta-mannosidase and beta-glucuronidase activities. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QDO67494.1 | 0.0 | 5 | 960 | 4 | 959 |
| QIU94545.1 | 0.0 | 1 | 960 | 1 | 958 |
| CBK68224.1 | 0.0 | 1 | 960 | 1 | 958 |
| QDH54516.1 | 0.0 | 1 | 960 | 1 | 958 |
| QRM98514.1 | 0.0 | 1 | 960 | 1 | 958 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7VQM_A | 1.10e-18 | 27 | 410 | 16 | 368 | ChainA, GH2 beta-galacturonate AqGalA [Aquimarina sp.],7VQM_B Chain B, GH2 beta-galacturonate AqGalA [Aquimarina sp.],7VQM_C Chain C, GH2 beta-galacturonate AqGalA [Aquimarina sp.],7VQM_D Chain D, GH2 beta-galacturonate AqGalA [Aquimarina sp.] |
| 3K4A_A | 1.35e-17 | 27 | 359 | 17 | 305 | Crystalstructure of selenomethionine substituted E. coli beta-glucuronidase [Escherichia coli K-12],3K4A_B Crystal structure of selenomethionine substituted E. coli beta-glucuronidase [Escherichia coli K-12] |
| 4JHZ_A | 4.07e-17 | 27 | 359 | 17 | 305 | Structureof E. coli beta-Glucuronidase bound with a novel, potent inhibitor 2-[4-(1,3-benzodioxol-5-ylmethyl)piperazin-1-yl]-N-[(1S,2S,5S)-2,5-dimethoxycyclohexyl]acetamide [Escherichia coli K-12],4JHZ_B Structure of E. coli beta-Glucuronidase bound with a novel, potent inhibitor 2-[4-(1,3-benzodioxol-5-ylmethyl)piperazin-1-yl]-N-[(1S,2S,5S)-2,5-dimethoxycyclohexyl]acetamide [Escherichia coli K-12] |
| 3LPF_A | 4.09e-17 | 27 | 359 | 17 | 305 | Structureof E. coli beta-Glucuronidase bound with a novel, potent inhibitor 1-((6,7-dimethyl-2-oxo-1,2-dihydroquinolin-3-yl)methyl)-1-(2-hydroxyethyl)-3-(3-methoxyphenyl)thiourea [Escherichia coli K-12],3LPF_B Structure of E. coli beta-Glucuronidase bound with a novel, potent inhibitor 1-((6,7-dimethyl-2-oxo-1,2-dihydroquinolin-3-yl)methyl)-1-(2-hydroxyethyl)-3-(3-methoxyphenyl)thiourea [Escherichia coli K-12],3LPG_A Structure of E. coli beta-Glucuronidase bound with a novel, potent inhibitor 3-(2-fluorophenyl)-1-(2-hydroxyethyl)-1-((6-methyl-2-oxo-1,2-dihydroquinolin-3-yl)methyl)urea [Escherichia coli K-12],3LPG_B Structure of E. coli beta-Glucuronidase bound with a novel, potent inhibitor 3-(2-fluorophenyl)-1-(2-hydroxyethyl)-1-((6-methyl-2-oxo-1,2-dihydroquinolin-3-yl)methyl)urea [Escherichia coli K-12],5CZK_A Structure of E. coli beta-glucuronidase bound with a novel, potent inhibitor 1-((6,8-dimethyl-2-oxo-1,2-dihydroquinolin-3-yl)methyl)-1-(2-hydroxyethyl)-3-(4-hydroxyphenyl)thiourea [Escherichia coli K-12],5CZK_B Structure of E. coli beta-glucuronidase bound with a novel, potent inhibitor 1-((6,8-dimethyl-2-oxo-1,2-dihydroquinolin-3-yl)methyl)-1-(2-hydroxyethyl)-3-(4-hydroxyphenyl)thiourea [Escherichia coli K-12] |
| 6JZ1_A | 5.37e-17 | 26 | 411 | 19 | 359 | Apostructure of b-glucuronidase from Ruminococcus gnavus at 1.7 Angstrom resolution [[Ruminococcus] gnavus],6JZ1_B Apo structure of b-glucuronidase from Ruminococcus gnavus at 1.7 Angstrom resolution [[Ruminococcus] gnavus],6JZ5_A b-glucuronidase from Ruminococcus gnavus in complex with D-glucuronic acid [[Ruminococcus] gnavus],6JZ5_B b-glucuronidase from Ruminococcus gnavus in complex with D-glucuronic acid [[Ruminococcus] gnavus],6JZ6_A b-glucuronidase from Ruminococcus gnavus in complex with C6-substituted uronic isofagomine [[Ruminococcus] gnavus],6JZ6_B b-glucuronidase from Ruminococcus gnavus in complex with C6-substituted uronic isofagomine [[Ruminococcus] gnavus],6JZ7_A b-glucuronidase from Ruminococcus gnavus in complex with N1-substituted uronic isofagomine [[Ruminococcus] gnavus],6JZ7_B b-glucuronidase from Ruminococcus gnavus in complex with N1-substituted uronic isofagomine [[Ruminococcus] gnavus],6JZ8_A b-glucuronidase from Ruminococcus gnavus in complex with D-glucaro 1,5-lactone [[Ruminococcus] gnavus],6JZ8_B b-glucuronidase from Ruminococcus gnavus in complex with D-glucaro 1,5-lactone [[Ruminococcus] gnavus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P05804 | 6.77e-16 | 27 | 359 | 15 | 303 | Beta-glucuronidase OS=Escherichia coli (strain K12) OX=83333 GN=uidA PE=1 SV=2 |
| P08236 | 6.71e-12 | 27 | 483 | 41 | 483 | Beta-glucuronidase OS=Homo sapiens OX=9606 GN=GUSB PE=1 SV=2 |
| Q5R5N6 | 1.16e-11 | 27 | 483 | 41 | 483 | Beta-glucuronidase OS=Pongo abelii OX=9601 GN=GUSB PE=2 SV=2 |
| O77695 | 4.58e-11 | 27 | 480 | 38 | 477 | Beta-glucuronidase (Fragment) OS=Chlorocebus aethiops OX=9534 GN=GUSB PE=2 SV=1 |
| P0C1Y0 | 3.14e-06 | 19 | 478 | 47 | 487 | Beta-galactosidase OS=Lactobacillus delbrueckii subsp. bulgaricus OX=1585 GN=lacZ PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
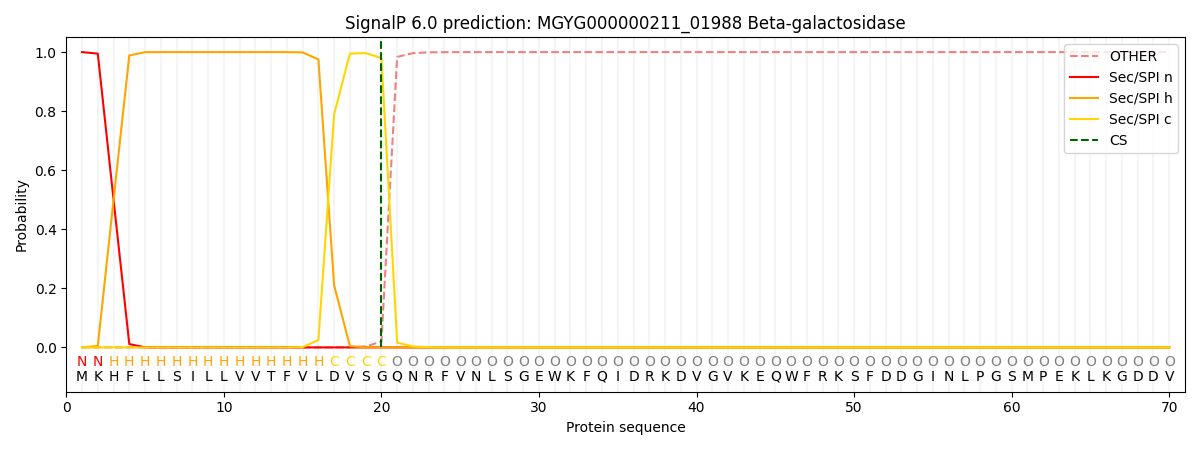
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000202 | 0.999238 | 0.000142 | 0.000144 | 0.000133 | 0.000129 |
