You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000227_04514
You are here: Home > Sequence: MGYG000000227_04514
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacillus sonorensis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Bacillales; Bacillaceae; Bacillus; Bacillus sonorensis | |||||||||||
| CAZyme ID | MGYG000000227_04514 | |||||||||||
| CAZy Family | GT2 | |||||||||||
| CAZyme Description | Phthiocerol synthesis polyketide synthase type I PpsD | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 11368; End: 16953 Strand: + | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd00833 | PKS | 0.0 | 9 | 433 | 1 | 421 | polyketide synthases (PKSs) polymerize simple fatty acids into a large variety of different products, called polyketides, by successive decarboxylating Claisen condensations. PKSs can be divided into 2 groups, modular type I PKSs consisting of one or more large multifunctional proteins and iterative type II PKSs, complexes of several monofunctional subunits. |
| COG3321 | PksD | 0.0 | 9 | 1096 | 4 | 1053 | Acyl transferase domain in polyketide synthase (PKS) enzymes [Secondary metabolites biosynthesis, transport and catabolism]. |
| smart00825 | PKS_KS | 4.22e-172 | 11 | 435 | 1 | 298 | Beta-ketoacyl synthase. The structure of beta-ketoacyl synthase is similar to that of the thiolase family and also chalcone synthase. The active site of beta-ketoacyl synthase is located between the N and C-terminal domains. |
| cd08955 | KR_2_FAS_SDR_x | 6.26e-130 | 1298 | 1691 | 8 | 376 | beta-ketoacyl reductase (KR) domain of fatty acid synthase (FAS), subgroup 2, complex (x). Ketoreductase, a module of the multidomain polyketide synthase, has 2 subdomains, each corresponding to a short-chain dehydrogenases/reductase (SDR) family monomer. The C-terminal subdomain catalyzes the NADPH-dependent reduction of the beta-carbonyl of a polyketide to a hydroxyl group, a step in the biosynthesis of polyketides, such as erythromycin. The N-terminal subdomain, an interdomain linker, is a truncated Rossmann fold which acts to stabilizes the catalytic subdomain. Unlike typical SDRs, the isolated domain does not oligomerizes but is composed of 2 subdomains, each resembling an SDR monomer. In some instances, as in porcine FAS, an enoyl reductase (a Rossman fold NAD binding domain of the MDR family) module is inserted between the sub-domains. The active site resembles that of typical SDRs, except that the usual positions of the catalytic asparagine and tyrosine are swapped, so that the canonical YXXXK motif changes to YXXXN. Modular polyketide synthases are multifunctional structures in which the makeup recapitulates that found in (and may have evolved from) fatty acid synthase. In some instances, such as porcine FAS , an enoyl reductase module is inserted between the sub-domains. Fatty acid synthesis occurs via the stepwise elongation of a chain (which is attached to acyl carrier protein, ACP) with 2-carbon units. Eukaryotic systems consists of large, multifunctional synthases (type I) while bacterial, type II systems, use single function proteins. Fungal fatty acid synthesis uses dodecamer of 6 alpha and 6 beta subunits. In mammalian type FAS cycles, ketoacyl synthase forms acetoacetyl-ACP which is reduced by the NADP-dependent beta-ketoacyl reductase (KR), forming beta-hydroxyacyl-ACP, which is in turn dehydrated by dehydratase to a beta-enoyl intermediate, which is reduced by NADP-dependent beta-enoyl reductase (ER). Polyketide syntheses also proceeds via the addition of 2-carbon units as in fatty acid synthesis. The complex SDR NADP binding motif, GGXGXXG, is often present, but is not strictly conserved in each instance of the module. This subfamily includes the KR domain of the Lyngbya majuscule Jam J, -K, and #L which are encoded on the jam gene cluster and are involved in the synthesis of the Jamaicamides (neurotoxins); Lyngbya majuscule Jam P belongs to a different KR_FAS_SDR_x subfamily. SDRs are a functionally diverse family of oxidoreductases that have a single domain with a structurally conserved Rossmann fold (alpha/beta folding pattern with a central beta-sheet), an NAD(P)(H)-binding region, and a structurally diverse C-terminal region. Classical SDRs are typically about 250 residues long, while extended SDRs are approximately 350 residues. Sequence identity between different SDR enzymes are typically in the 15-30% range, but the enzymes share the Rossmann fold NAD-binding motif and characteristic NAD-binding and catalytic sequence patterns. These enzymes catalyze a wide range of activities including the metabolism of steroids, cofactors, carbohydrates, lipids, aromatic compounds, and amino acids, and act in redox sensing. Classical SDRs have an TGXXX[AG]XG cofactor binding motif and a YXXXK active site motif, with the Tyr residue of the active site motif serving as a critical catalytic residue (Tyr-151, human prostaglandin dehydrogenase (PGDH) numbering). In addition to the Tyr and Lys, there is often an upstream Ser (Ser-138, PGDH numbering) and/or an Asn (Asn-107, PGDH numbering) contributing to the active site; while substrate binding is in the C-terminal region, which determines specificity. The standard reaction mechanism is a 4-pro-S hydride transfer and proton relay involving the conserved Tyr and Lys, a water molecule stabilized by Asn, and nicotinamide. Extended SDRs have additional elements in the C-terminal region, and typically have a TGXXGXXG cofactor binding motif. Complex (multidomain) SDRs such as ketoreductase domains of fatty acid synthase have a GGXGXXG NAD(P)-binding motif and an altered active site motif (YXXXN). Fungal type KRs have a TGXXXGX(1-2)G NAD(P)-binding motif. Some atypical SDRs have lost catalytic activity and/or have an unusual NAD(P)-binding motif and missing or unusual active site residues. Reactions catalyzed within the SDR family include isomerization, decarboxylation, epimerization, C=N bond reduction, dehydratase activity, dehalogenation, Enoyl-CoA reduction, and carbonyl-alcohol oxidoreduction. |
| smart00827 | PKS_AT | 3.46e-127 | 552 | 847 | 1 | 297 | Acyl transferase domain in polyketide synthase (PKS) enzymes. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BAZ00088.1 | 3.35e-144 | 11 | 918 | 1214 | 2111 |
| BAZ75991.1 | 3.35e-144 | 11 | 918 | 1214 | 2111 |
| BAY30132.1 | 5.98e-144 | 11 | 903 | 1216 | 2103 |
| BAY90071.1 | 2.52e-143 | 11 | 903 | 1213 | 2100 |
| AFY93865.1 | 1.25e-121 | 3 | 903 | 953 | 1869 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4MZ0_A | 5.04e-229 | 3 | 895 | 34 | 935 | ChainA, CurL [Moorena producens 3L],4MZ0_B Chain B, CurL [Moorena producens 3L] |
| 7M7E_A | 6.77e-211 | 2 | 1827 | 26 | 1490 | ChainA, 6-deoxyerythronolide-B synthase EryA2, modules 3 and 4,EryAI,6-deoxyerythronolide-B synthase EryA3, modules 5 and 6 chimera [Saccharopolyspora erythraea],7M7E_B Chain B, 6-deoxyerythronolide-B synthase EryA2, modules 3 and 4,EryAI,6-deoxyerythronolide-B synthase EryA3, modules 5 and 6 chimera [Saccharopolyspora erythraea] |
| 7M7I_A | 4.53e-206 | 2 | 1861 | 26 | 1521 | ChainA, EryAI [Saccharopolyspora erythraea],7M7I_B Chain B, EryAI [Saccharopolyspora erythraea] |
| 7M7J_A | 1.21e-205 | 2 | 1861 | 26 | 1521 | ChainA, EryAI [Saccharopolyspora erythraea],7M7J_B Chain B, EryAI [Saccharopolyspora erythraea] |
| 7M7F_A | 7.00e-204 | 2 | 1827 | 26 | 1497 | ChainA, EryAI,6-deoxyerythronolide-B synthase EryA3, modules 5 and 6 chimera [Saccharopolyspora erythraea],7M7F_B Chain B, EryAI,6-deoxyerythronolide-B synthase EryA3, modules 5 and 6 chimera [Saccharopolyspora erythraea],7M7G_A Chain A, EryAI,6-deoxyerythronolide-B synthase EryA3, modules 5 and 6 chimera [Saccharopolyspora erythraea],7M7G_B Chain B, EryAI,6-deoxyerythronolide-B synthase EryA3, modules 5 and 6 chimera [Saccharopolyspora erythraea],7M7H_A Chain A, EryAI,6-deoxyerythronolide-B synthase EryA3, modules 5 and 6 chimera [Saccharopolyspora erythraea],7M7H_B Chain B, EryAI,6-deoxyerythronolide-B synthase EryA3, modules 5 and 6 chimera [Saccharopolyspora erythraea] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P9WQE2 | 1.89e-282 | 4 | 1820 | 32 | 1785 | Phenolphthiocerol/phthiocerol polyketide synthase subunit C OS=Mycobacterium tuberculosis (strain CDC 1551 / Oshkosh) OX=83331 GN=ppsD PE=3 SV=1 |
| Q7TXL7 | 1.89e-282 | 4 | 1820 | 32 | 1785 | Phenolphthiocerol/phthiocerol polyketide synthase subunit C OS=Mycobacterium bovis (strain ATCC BAA-935 / AF2122/97) OX=233413 GN=ppsD PE=1 SV=1 |
| P9WQE3 | 1.89e-282 | 4 | 1820 | 32 | 1785 | Phenolphthiocerol/phthiocerol polyketide synthase subunit D OS=Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) OX=83332 GN=ppsD PE=1 SV=1 |
| P9WQE7 | 9.93e-242 | 9 | 1820 | 102 | 1836 | Phenolphthiocerol/phthiocerol polyketide synthase subunit A OS=Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) OX=83332 GN=ppsA PE=1 SV=1 |
| P9WQE6 | 1.38e-241 | 9 | 1820 | 102 | 1836 | Phenolphthiocerol/phthiocerol polyketide synthase subunit A OS=Mycobacterium tuberculosis (strain CDC 1551 / Oshkosh) OX=83331 GN=ppsA PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
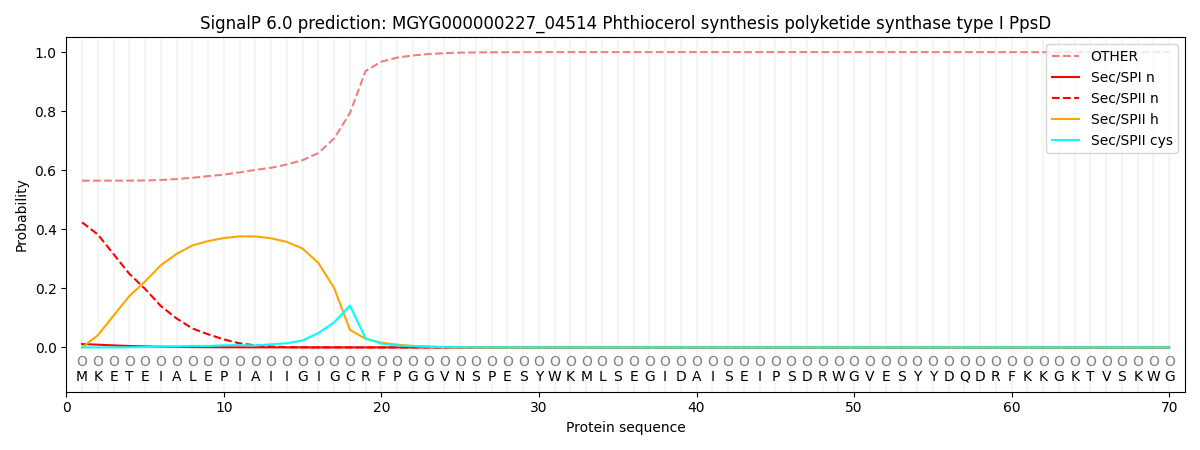
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.565366 | 0.010167 | 0.423759 | 0.000097 | 0.000070 | 0.000547 |
