You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000414_00493
You are here: Home > Sequence: MGYG000000414_00493
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
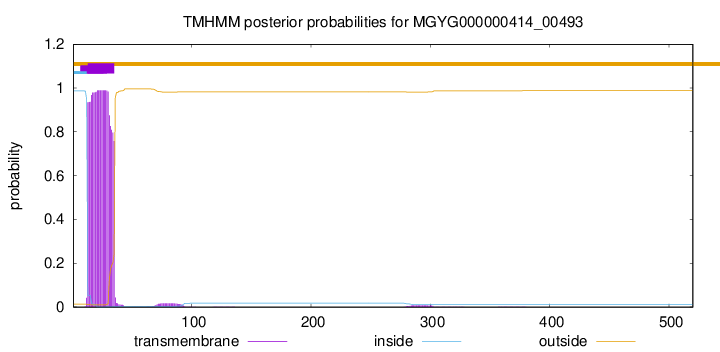
TMHMM annotations
Basic Information help
| Species | Alistipes sp900546065 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Rikenellaceae; Alistipes; Alistipes sp900546065 | |||||||||||
| CAZyme ID | MGYG000000414_00493 | |||||||||||
| CAZy Family | CE3 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 54925; End: 56487 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE3 | 51 | 239 | 1e-38 | 0.9845360824742269 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd01833 | XynB_like | 7.81e-21 | 51 | 239 | 3 | 157 | SGNH_hydrolase subfamily, similar to Ruminococcus flavefaciens XynB. Most likely a secreted hydrolase with xylanase activity. SGNH hydrolases are a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the Ser-His-Asp(Glu) triad found in other serine hydrolases. |
| COG0657 | Aes | 5.38e-20 | 210 | 470 | 9 | 292 | Acetyl esterase/lipase [Lipid transport and metabolism]. |
| cd00229 | SGNH_hydrolase | 3.65e-16 | 51 | 238 | 1 | 187 | SGNH_hydrolase, or GDSL_hydrolase, is a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the typical Ser-His-Asp(Glu) triad from other serine hydrolases, but may lack the carboxlic acid. |
| pfam13472 | Lipase_GDSL_2 | 7.53e-16 | 53 | 230 | 1 | 176 | GDSL-like Lipase/Acylhydrolase family. This family of presumed lipases and related enzymes are similar to pfam00657. |
| pfam07859 | Abhydrolase_3 | 2.88e-14 | 285 | 464 | 1 | 205 | alpha/beta hydrolase fold. This catalytic domain is found in a very wide range of enzymes. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BAX78799.1 | 2.13e-144 | 50 | 494 | 39 | 494 |
| ATC65549.1 | 9.89e-127 | 41 | 494 | 32 | 492 |
| QOV88633.1 | 1.23e-57 | 21 | 239 | 9 | 222 |
| QDT00894.1 | 7.81e-47 | 246 | 496 | 26 | 285 |
| QNN20991.1 | 2.35e-43 | 51 | 243 | 32 | 226 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5AO9_A | 5.44e-46 | 253 | 494 | 16 | 278 | Thestructure of a novel thermophilic esterase from the Planctomycetes species, Thermogutta terrifontis, Est2-native [Thermogutta terrifontis],5AOA_A The structure of a novel thermophilic esterase from the Planctomycetes species, Thermogutta terrifontis, Est2-Propionate bound [Thermogutta terrifontis],5AOB_A The structure of a novel thermophilic esterase from the Planctomycetes species, Thermogutta terrifontis, Est2-butyrate bound [Thermogutta terrifontis],5AOC_A The structure of a novel thermophilic esterase from the Planctomycetes species, Thermogutta terrifontis, Est2-valerate bound [Thermogutta terrifontis] |
| 7BFN_A | 5.59e-46 | 253 | 494 | 17 | 279 | ChainA, Esterase [Thermogutta terrifontis] |
| 7BFO_A | 2.93e-45 | 253 | 494 | 17 | 279 | ChainA, Esterase [Thermogutta terrifontis],7BFR_A Chain A, Esterase [Thermogutta terrifontis],7BFT_A Chain A, Esterase [Thermogutta terrifontis],7BFU_A Chain A, Esterase [Thermogutta terrifontis],7BFV_A Chain A, Esterase [Thermogutta terrifontis] |
| 1EVQ_A | 2.43e-15 | 231 | 476 | 25 | 295 | THECRYSTAL STRUCTURE OF THE THERMOPHILIC CARBOXYLESTERASE EST2 FROM ALICYCLOBACILLUS ACIDOCALDARIUS [Alicyclobacillus acidocaldarius] |
| 1QZ3_A | 5.90e-15 | 231 | 476 | 25 | 295 | CRYSTALSTRUCTURE OF MUTANT M211S/R215L OF CARBOXYLESTERASE EST2 COMPLEXED WITH HEXADECANESULFONATE [Alicyclobacillus acidocaldarius],1U4N_A Crystal Structure Analysis of the M211S/R215L EST2 mutant [Alicyclobacillus acidocaldarius] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P96402 | 2.94e-12 | 283 | 456 | 156 | 362 | Esterase LipC OS=Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) OX=83332 GN=lipC PE=1 SV=1 |
| Q9US38 | 8.50e-12 | 270 | 396 | 88 | 217 | AB hydrolase superfamily protein C1039.03 OS=Schizosaccharomyces pombe (strain 972 / ATCC 24843) OX=284812 GN=SPAC1039.03 PE=3 SV=1 |
| A0A0G3FWY4 | 8.47e-11 | 285 | 374 | 76 | 163 | Probable N-octanoylanthranilate hydrolase AqdA1 OS=Rhodococcus erythropolis OX=1833 GN=aqdA1 PE=1 SV=1 |
| Q50681 | 4.17e-10 | 263 | 449 | 161 | 374 | Probable carboxylic ester hydrolase LipM OS=Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) OX=83332 GN=lipM PE=1 SV=1 |
| P9WK87 | 4.21e-10 | 261 | 453 | 62 | 268 | Carboxylesterase NlhH OS=Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) OX=83332 GN=nlhH PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
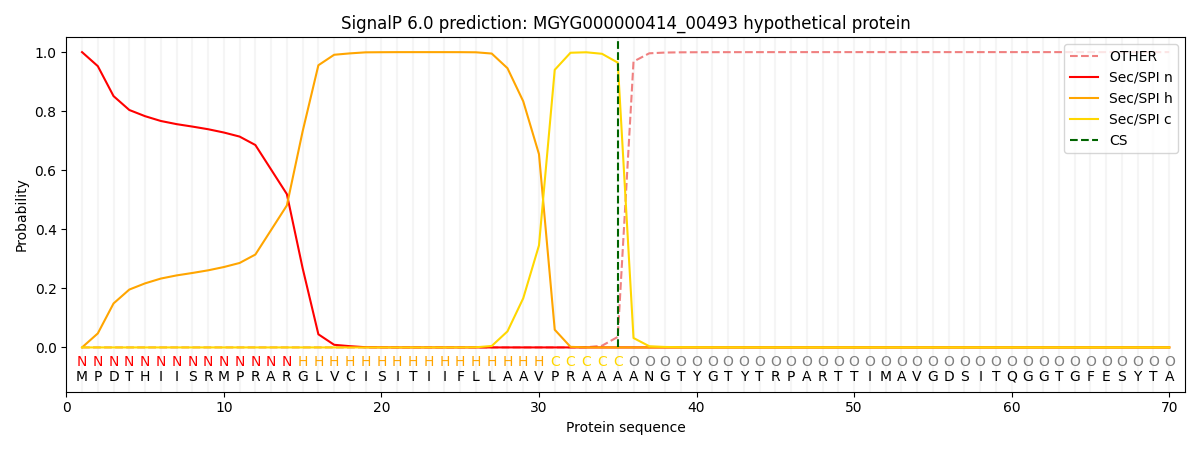
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000489 | 0.998729 | 0.000256 | 0.000172 | 0.000169 | 0.000151 |