You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000424_00798
You are here: Home > Sequence: MGYG000000424_00798
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | CAG-353 sp900768995 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Ruminococcaceae; CAG-353; CAG-353 sp900768995 | |||||||||||
| CAZyme ID | MGYG000000424_00798 | |||||||||||
| CAZy Family | GH5 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 55339; End: 57384 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH5 | 297 | 635 | 8.8e-120 | 0.9967741935483871 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam00150 | Cellulase | 3.07e-47 | 296 | 630 | 1 | 272 | Cellulase (glycosyl hydrolase family 5). |
| COG2730 | BglC | 5.40e-36 | 305 | 643 | 47 | 378 | Aryl-phospho-beta-D-glucosidase BglC, GH1 family [Carbohydrate transport and metabolism]. |
| smart00637 | CBD_II | 8.50e-09 | 158 | 237 | 2 | 77 | CBD_II domain. |
| pfam00553 | CBM_2 | 6.66e-06 | 150 | 239 | 1 | 86 | Cellulose binding domain. Two tryptophan residues are involved in cellulose binding. Cellulose binding domain found in bacteria. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CUH91791.1 | 1.74e-254 | 144 | 679 | 33 | 575 |
| AGS52139.1 | 1.20e-237 | 155 | 679 | 126 | 659 |
| ADD61853.1 | 3.36e-236 | 276 | 681 | 97 | 503 |
| QWT53501.1 | 2.73e-235 | 276 | 681 | 97 | 503 |
| QNL98527.1 | 7.78e-235 | 276 | 681 | 97 | 503 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1ECE_A | 1.52e-66 | 287 | 648 | 5 | 343 | AcidothermusCellulolyticus Endocellulase E1 Catalytic Domain In Complex With A Cellotetraose [Acidothermus cellulolyticus],1ECE_B Acidothermus Cellulolyticus Endocellulase E1 Catalytic Domain In Complex With A Cellotetraose [Acidothermus cellulolyticus] |
| 1VRX_A | 1.12e-65 | 287 | 648 | 5 | 343 | ChainA, ENDOCELLULASE E1 FROM A. CELLULOLYTICUS [Acidothermus cellulolyticus],1VRX_B Chain B, ENDOCELLULASE E1 FROM A. CELLULOLYTICUS [Acidothermus cellulolyticus] |
| 4TUF_A | 4.63e-55 | 294 | 657 | 8 | 344 | Catalyticdomain of the major endoglucanase from Xanthomonas campestris pv. campestris [Xanthomonas campestris pv. campestris str. ATCC 33913],4TUF_B Catalytic domain of the major endoglucanase from Xanthomonas campestris pv. campestris [Xanthomonas campestris pv. campestris str. ATCC 33913],4TUF_C Catalytic domain of the major endoglucanase from Xanthomonas campestris pv. campestris [Xanthomonas campestris pv. campestris str. ATCC 33913],4TUF_D Catalytic domain of the major endoglucanase from Xanthomonas campestris pv. campestris [Xanthomonas campestris pv. campestris str. ATCC 33913] |
| 3VVG_A | 3.85e-54 | 305 | 656 | 30 | 376 | TheCrystal Structure of Cellulase-Inhibitor Complex. [Pyrococcus horikoshii OT3],3VVG_B The Crystal Structure of Cellulase-Inhibitor Complex. [Pyrococcus horikoshii OT3],3VVG_C The Crystal Structure of Cellulase-Inhibitor Complex. [Pyrococcus horikoshii OT3],3W6L_A Contribution of disulfide bond toward thermostability in hyperthermostable endocellulase [Pyrococcus horikoshii OT3],3W6L_B Contribution of disulfide bond toward thermostability in hyperthermostable endocellulase [Pyrococcus horikoshii OT3],3W6L_C Contribution of disulfide bond toward thermostability in hyperthermostable endocellulase [Pyrococcus horikoshii OT3],4DM1_A Contribution of disulfide bond toward thermostability in hyperthermostable endocellulase [Pyrococcus horikoshii OT3],4DM1_B Contribution of disulfide bond toward thermostability in hyperthermostable endocellulase [Pyrococcus horikoshii OT3],4DM1_C Contribution of disulfide bond toward thermostability in hyperthermostable endocellulase [Pyrococcus horikoshii OT3] |
| 3W6M_A | 5.33e-54 | 305 | 656 | 30 | 376 | Contributionof disulfide bond toward thermostability in hyperthermostable endocellulase [Pyrococcus horikoshii OT3],3W6M_B Contribution of disulfide bond toward thermostability in hyperthermostable endocellulase [Pyrococcus horikoshii OT3],3W6M_C Contribution of disulfide bond toward thermostability in hyperthermostable endocellulase [Pyrococcus horikoshii OT3],4DM2_A Contribution of disulfide bond toward thermostability in hyperthermostable endocellulase [Pyrococcus horikoshii OT3],4DM2_B Contribution of disulfide bond toward thermostability in hyperthermostable endocellulase [Pyrococcus horikoshii OT3],4DM2_C Contribution of disulfide bond toward thermostability in hyperthermostable endocellulase [Pyrococcus horikoshii OT3] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P10474 | 1.07e-180 | 279 | 679 | 621 | 1021 | Endoglucanase/exoglucanase B OS=Caldicellulosiruptor saccharolyticus OX=44001 GN=celB PE=3 SV=1 |
| Q05332 | 2.88e-151 | 285 | 681 | 42 | 470 | Endoglucanase G OS=Acetivibrio thermocellus (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) OX=203119 GN=celG PE=3 SV=1 |
| P50400 | 3.17e-147 | 276 | 680 | 34 | 438 | Endoglucanase D OS=Cellulomonas fimi OX=1708 GN=cenD PE=3 SV=1 |
| P04956 | 7.99e-135 | 281 | 679 | 35 | 467 | Endoglucanase B OS=Acetivibrio thermocellus (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) OX=203119 GN=celB PE=3 SV=1 |
| P23548 | 5.56e-78 | 281 | 656 | 32 | 382 | Endoglucanase OS=Paenibacillus polymyxa OX=1406 PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
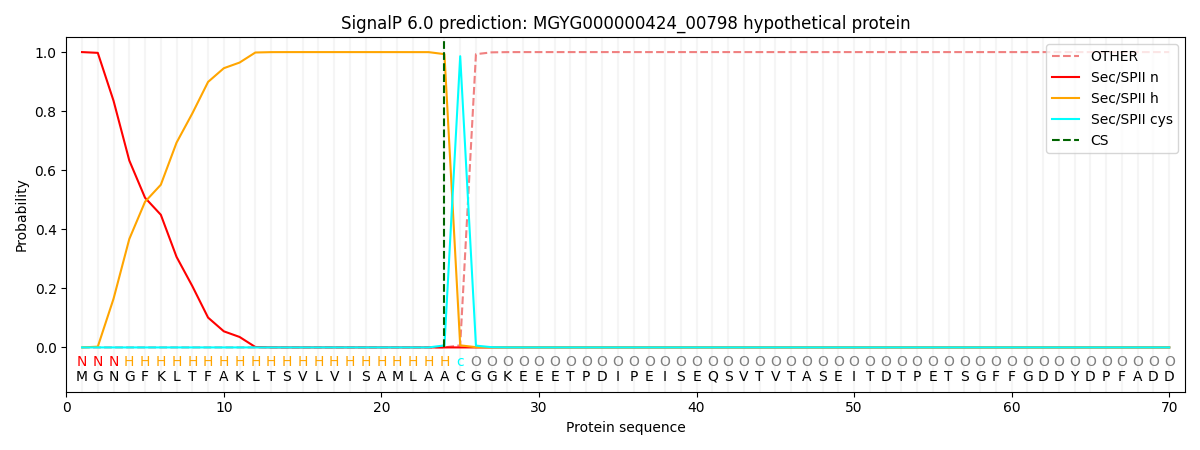
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000000 | 0.000005 | 1.000029 | 0.000000 | 0.000000 | 0.000000 |
