You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000452_01651
You are here: Home > Sequence: MGYG000000452_01651
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Barnesiella sp002159975 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Barnesiellaceae; Barnesiella; Barnesiella sp002159975 | |||||||||||
| CAZyme ID | MGYG000000452_01651 | |||||||||||
| CAZy Family | GH29 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 6027; End: 7526 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH29 | 46 | 358 | 3.1e-67 | 0.869942196531792 |
| CBM32 | 372 | 486 | 5.7e-20 | 0.8306451612903226 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3669 | AfuC | 4.10e-47 | 41 | 499 | 4 | 430 | Alpha-L-fucosidase [Carbohydrate transport and metabolism]. |
| smart00812 | Alpha_L_fucos | 2.40e-30 | 63 | 355 | 31 | 327 | Alpha-L-fucosidase. O-Glycosyl hydrolases (EC 3.2.1.-) are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycosyl hydrolases, based on sequence similarity, has led to the definition of 85 different families. This classification is available on the CAZy (CArbohydrate-Active EnZymes) web site. Because the fold of proteins is better conserved than their sequences, some of the families can be grouped in 'clans'. Family 29 encompasses alpha-L-fucosidases, which is a lysosomal enzyme responsible for hydrolyzing the alpha-1,6-linked fucose joined to the reducing-end N-acetylglucosamine of the carbohydrate moieties of glycoproteins. Deficiency of alpha-L-fucosidase results in the lysosomal storage disease fucosidosis. |
| pfam01120 | Alpha_L_fucos | 2.29e-28 | 59 | 355 | 44 | 325 | Alpha-L-fucosidase. |
| pfam00754 | F5_F8_type_C | 1.11e-15 | 371 | 487 | 1 | 115 | F5/8 type C domain. This domain is also known as the discoidin (DS) domain family. |
| cd00057 | FA58C | 1.73e-05 | 390 | 485 | 30 | 129 | Substituted updates: Jan 31, 2002 |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AHF11782.1 | 0.0 | 1 | 499 | 1 | 499 |
| QFQ12303.1 | 8.12e-141 | 6 | 499 | 2 | 492 |
| QFQ12382.1 | 6.53e-140 | 36 | 499 | 32 | 492 |
| AUI56213.1 | 9.39e-136 | 10 | 499 | 10 | 509 |
| QUB82373.1 | 2.66e-135 | 10 | 499 | 10 | 509 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6ORG_A | 1.21e-68 | 46 | 499 | 9 | 448 | Crystalstructure of SpGH29 [Streptococcus pneumoniae TIGR4],6ORG_B Crystal structure of SpGH29 [Streptococcus pneumoniae TIGR4] |
| 6OR4_A | 3.25e-68 | 46 | 499 | 9 | 448 | Crystalstructure of SpGH29 [Streptococcus pneumoniae TIGR4],6OR4_B Crystal structure of SpGH29 [Streptococcus pneumoniae TIGR4],6ORH_A Crystal structure of SpGH29 [Streptococcus pneumoniae TIGR4],6ORH_B Crystal structure of SpGH29 [Streptococcus pneumoniae TIGR4] |
| 6ORF_A | 3.33e-68 | 46 | 499 | 9 | 448 | Crystalstructure of SpGH29 [Streptococcus pneumoniae TIGR4],6ORF_B Crystal structure of SpGH29 [Streptococcus pneumoniae TIGR4] |
| 3UES_A | 2.46e-67 | 48 | 494 | 19 | 471 | Crystalstructure of alpha-1,3/4-fucosidase from Bifidobacterium longum subsp. infantis complexed with deoxyfuconojirimycin [Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 = DSM 20088],3UES_B Crystal structure of alpha-1,3/4-fucosidase from Bifidobacterium longum subsp. infantis complexed with deoxyfuconojirimycin [Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 = DSM 20088] |
| 3UET_A | 1.85e-66 | 48 | 494 | 19 | 471 | Crystalstructure of alpha-1,3/4-fucosidase from Bifidobacterium longum subsp. infantis D172A/E217A mutant complexed with lacto-N-fucopentaose II [Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 = DSM 20088],3UET_B Crystal structure of alpha-1,3/4-fucosidase from Bifidobacterium longum subsp. infantis D172A/E217A mutant complexed with lacto-N-fucopentaose II [Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 = DSM 20088] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q7XUR3 | 7.53e-70 | 9 | 499 | 3 | 476 | Putative alpha-L-fucosidase 1 OS=Oryza sativa subsp. japonica OX=39947 GN=Os04g0560400 PE=3 SV=2 |
| Q8GW72 | 6.13e-69 | 41 | 499 | 31 | 477 | Alpha-L-fucosidase 1 OS=Arabidopsis thaliana OX=3702 GN=FUC1 PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as SP
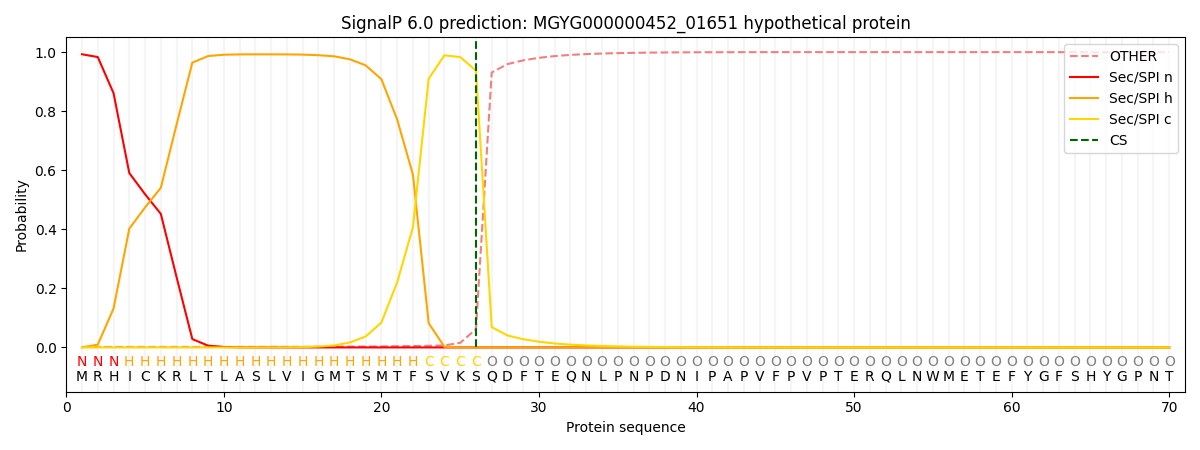
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.003535 | 0.989240 | 0.006174 | 0.000518 | 0.000254 | 0.000217 |
