You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000604_00378
You are here: Home > Sequence: MGYG000000604_00378
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | UBA1829 sp900549415 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Verrucomicrobiota; Lentisphaeria; Victivallales; UBA1829; UBA1829; UBA1829 sp900549415 | |||||||||||
| CAZyme ID | MGYG000000604_00378 | |||||||||||
| CAZy Family | GT2 | |||||||||||
| CAZyme Description | Undecaprenyl-phosphate 4-deoxy-4-formamido-L-arabinose transferase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 146664; End: 148073 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT2 | 88 | 255 | 6e-33 | 0.9705882352941176 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd06434 | GT2_HAS | 9.79e-48 | 87 | 320 | 2 | 231 | Hyaluronan synthases catalyze polymerization of hyaluronan. Hyaluronan synthases (HASs) are bi-functional glycosyltransferases that catalyze polymerization of hyaluronan. HASs transfer both GlcUA and GlcNAc in beta-(1,3) and beta-(1,4) linkages, respectively to the hyaluronan chain using UDP-GlcNAc and UDP-GlcUA as substrates. HA is made as a free glycan, not attached to a protein or lipid. HASs do not need a primer for HA synthesis; they initiate HA biosynthesis de novo with only UDP-GlcNAc, UDP-GlcUA, and Mg2+. Hyaluronan (HA) is a linear heteropolysaccharide composed of (1-3)-linked beta-D-GlcUA-beta-D-GlcNAc disaccharide repeats. It can be found in vertebrates and a few microbes and is typically on the cell surface or in the extracellular space, but is also found inside mammalian cells. Hyaluronan has several physiochemical and biological functions such as space filling, lubrication, and providing a hydrated matrix through which cells can migrate. |
| cd06423 | CESA_like | 4.10e-45 | 89 | 277 | 1 | 180 | CESA_like is the cellulose synthase superfamily. The cellulose synthase (CESA) superfamily includes a wide variety of glycosyltransferase family 2 enzymes that share the common characteristic of catalyzing the elongation of polysaccharide chains. The members include cellulose synthase catalytic subunit, chitin synthase, glucan biosynthesis protein and other families of CESA-like proteins. Cellulose synthase catalyzes the polymerization reaction of cellulose, an aggregate of unbranched polymers of beta-1,4-linked glucose residues in plants, most algae, some bacteria and fungi, and even some animals. In bacteria, algae and lower eukaryotes, there is a second unrelated type of cellulose synthase (Type II), which produces acylated cellulose, a derivative of cellulose. Chitin synthase catalyzes the incorporation of GlcNAc from substrate UDP-GlcNAc into chitin, which is a linear homopolymer of beta-(1,4)-linked GlcNAc residues and Glucan Biosynthesis protein catalyzes the elongation of beta-1,2 polyglucose chains of Glucan. |
| COG1215 | BcsA | 2.50e-41 | 46 | 438 | 10 | 393 | Glycosyltransferase, catalytic subunit of cellulose synthase and poly-beta-1,6-N-acetylglucosamine synthase [Cell motility]. |
| cd06439 | CESA_like_1 | 8.21e-37 | 60 | 300 | 3 | 227 | CESA_like_1 is a member of the cellulose synthase (CESA) superfamily. This is a subfamily of cellulose synthase (CESA) superfamily. CESA superfamily includes a wide variety of glycosyltransferase family 2 enzymes that share the common characteristic of catalyzing the elongation of polysaccharide chains. The members of the superfamily include cellulose synthase catalytic subunit, chitin synthase, glucan biosynthesis protein and other families of CESA-like proteins. |
| PRK11204 | PRK11204 | 3.35e-35 | 61 | 388 | 23 | 352 | N-glycosyltransferase; Provisional |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CBX26966.1 | 8.56e-107 | 73 | 449 | 89 | 464 |
| BCS52180.1 | 6.47e-103 | 12 | 439 | 22 | 439 |
| QED36688.1 | 5.67e-102 | 2 | 440 | 49 | 483 |
| AQV02755.1 | 1.52e-101 | 1 | 439 | 38 | 475 |
| AOY60673.1 | 1.52e-101 | 1 | 439 | 38 | 475 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7SP7_A | 5.55e-23 | 6 | 330 | 6 | 356 | ChainA, Hyaluronan synthase [Paramecium bursaria Chlorella virus CZ-2] |
| 7SP6_A | 2.39e-22 | 6 | 330 | 6 | 356 | ChainA, Hyaluronan synthase [Paramecium bursaria Chlorella virus CZ-2],7SP8_A Chain A, Hyaluronan synthase [Paramecium bursaria Chlorella virus CZ-2],7SP9_A Chain A, Hyaluronan synthase [Paramecium bursaria Chlorella virus CZ-2],7SPA_A Chain A, Hyaluronan synthase [Paramecium bursaria Chlorella virus CZ-2] |
| 6YV7_B | 1.06e-12 | 85 | 282 | 42 | 226 | MannosyltransferasePcManGT from Pyrobaculum calidifontis [Pyrobaculum calidifontis JCM 11548],6YV8_B Mannosyltransferase PcManGT from Pyrobaculum calidifontis in complex with GDP and Mn2+ [Pyrobaculum calidifontis JCM 11548],6YV9_A Mannosyltransferase PcManGT from Pyrobaculum calidifontis in complex with GDP-Man and Mn2+ [Pyrobaculum calidifontis JCM 11548] |
| 6YV7_A | 1.07e-12 | 85 | 282 | 43 | 227 | MannosyltransferasePcManGT from Pyrobaculum calidifontis [Pyrobaculum calidifontis JCM 11548],6YV8_A Mannosyltransferase PcManGT from Pyrobaculum calidifontis in complex with GDP and Mn2+ [Pyrobaculum calidifontis JCM 11548],6YV9_B Mannosyltransferase PcManGT from Pyrobaculum calidifontis in complex with GDP-Man and Mn2+ [Pyrobaculum calidifontis JCM 11548] |
| 6P61_A | 3.30e-11 | 85 | 217 | 13 | 142 | Structureof a Glycosyltransferase from Leptospira borgpetersenii serovar Hardjo-bovis (strain JB197) [Leptospira borgpetersenii serovar Hardjo-bovis str. JB197],6P61_B Structure of a Glycosyltransferase from Leptospira borgpetersenii serovar Hardjo-bovis (strain JB197) [Leptospira borgpetersenii serovar Hardjo-bovis str. JB197],6P61_C Structure of a Glycosyltransferase from Leptospira borgpetersenii serovar Hardjo-bovis (strain JB197) [Leptospira borgpetersenii serovar Hardjo-bovis str. JB197],6P61_D Structure of a Glycosyltransferase from Leptospira borgpetersenii serovar Hardjo-bovis (strain JB197) [Leptospira borgpetersenii serovar Hardjo-bovis str. JB197] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P04341 | 1.12e-41 | 80 | 463 | 44 | 418 | N-acetylglucosaminyltransferase OS=Rhizobium meliloti (strain 1021) OX=266834 GN=nodC PE=3 SV=1 |
| P72334 | 1.20e-39 | 49 | 442 | 9 | 398 | N-acetylglucosaminyltransferase OS=Rhizobium sp. (strain N33) OX=103798 GN=nodC PE=3 SV=1 |
| P04340 | 5.26e-39 | 80 | 441 | 44 | 396 | N-acetylglucosaminyltransferase OS=Rhizobium leguminosarum bv. viciae OX=387 GN=nodC PE=3 SV=1 |
| Q07755 | 3.08e-38 | 85 | 439 | 48 | 394 | N-acetylglucosaminyltransferase OS=Azorhizobium caulinodans (strain ATCC 43989 / DSM 5975 / JCM 20966 / LMG 6465 / NBRC 14845 / NCIMB 13405 / ORS 571) OX=438753 GN=nodC PE=3 SV=2 |
| P50357 | 4.01e-37 | 75 | 441 | 39 | 397 | N-acetylglucosaminyltransferase OS=Sinorhizobium fredii (strain NBRC 101917 / NGR234) OX=394 GN=nodC PE=3 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
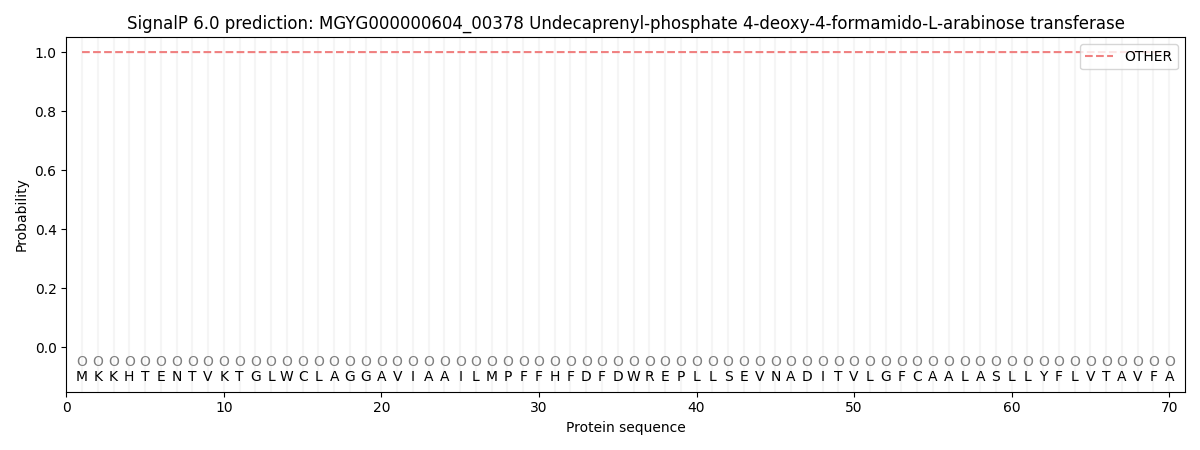
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 1.000048 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |
TMHMM Annotations download full data without filtering help
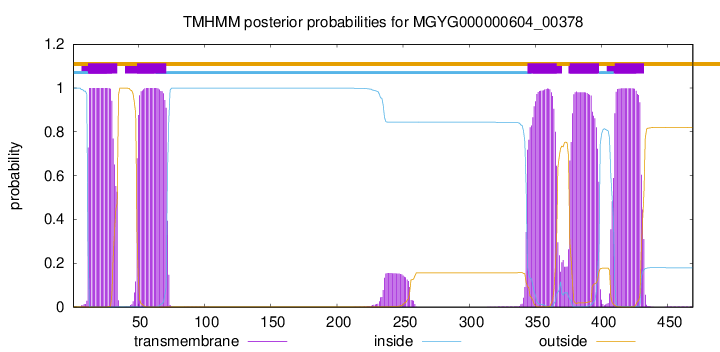
| start | end |
|---|---|
| 12 | 34 |
| 49 | 71 |
| 344 | 366 |
| 376 | 398 |
| 410 | 432 |
