You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000673_01551
You are here: Home > Sequence: MGYG000000673_01551
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | CAG-873 sp004552485 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Muribaculaceae; CAG-873; CAG-873 sp004552485 | |||||||||||
| CAZyme ID | MGYG000000673_01551 | |||||||||||
| CAZy Family | GH27 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 4249; End: 6786 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH27 | 172 | 416 | 1.7e-27 | 0.9388646288209607 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd14792 | GH27 | 2.30e-17 | 37 | 323 | 2 | 243 | glycosyl hydrolase family 27 (GH27). GH27 enzymes occur in eukaryotes, prokaryotes, and archaea with a wide range of hydrolytic activities, including alpha-glucosidase (glucoamylase and sucrase-isomaltase), alpha-N-acetylgalactosaminidase, and 3-alpha-isomalto-dextranase. All GH27 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH27 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| pfam17801 | Melibiase_C | 1.88e-11 | 367 | 437 | 5 | 74 | Alpha galactosidase C-terminal beta sandwich domain. This domain is found at the C-terminus of alpha galactosidase enzymes. |
| pfam16411 | SusF_SusE | 5.49e-08 | 449 | 611 | 1 | 139 | Outer membrane protein SusF_SusE. SusE and SusF are two outer membrane proteins composed of tandem starch specific carbohydrate binding modules (CBMs) with no enzymatic activity. They are are likely to play an important role in starch metabolism in Bacteroides. It has been speculated that they could compete for starch in the human intestinal tract by sequestering starch at the bacterial surface and away from competitors. SusE has higher affinity for starch compared to SusF. |
| PLN02808 | PLN02808 | 6.09e-06 | 37 | 440 | 33 | 386 | alpha-galactosidase |
| cd14791 | GH36 | 1.12e-04 | 35 | 219 | 2 | 191 | glycosyl hydrolase family 36 (GH36). GH36 enzymes occur in prokaryotes, eukaryotes, and archaea with a wide range of hydrolytic activities, including alpha-galactosidase, alpha-N-acetylgalactosaminidase, stachyose synthase, and raffinose synthase. All GH36 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH36 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUR46778.1 | 2.49e-151 | 76 | 436 | 1 | 365 |
| QUT92727.1 | 1.79e-146 | 25 | 436 | 24 | 440 |
| ALJ61711.1 | 2.53e-146 | 25 | 436 | 24 | 440 |
| QDM09865.1 | 3.02e-116 | 12 | 440 | 8 | 446 |
| QUT79841.1 | 4.34e-116 | 22 | 440 | 8 | 436 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5AWO_A | 3.75e-92 | 26 | 475 | 27 | 503 | Arthrobacterglobiformis T6 isomalto-dextranse [Arthrobacter globiformis],5AWP_A Arthrobacter globiformis T6 isomalto-dextranase complexed with isomaltose [Arthrobacter globiformis],5AWQ_A Arthrobacter globiformis T6 isomalto-dextranse complexed with panose [Arthrobacter globiformis] |
| 4NX0_A | 5.48e-18 | 37 | 439 | 26 | 442 | Crystalstructure of Abp-WT, a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NX0_B Crystal structure of Abp-WT, a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NX0_C Crystal structure of Abp-WT, a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NX0_D Crystal structure of Abp-WT, a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NX0_E Crystal structure of Abp-WT, a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NX0_F Crystal structure of Abp-WT, a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NX0_G Crystal structure of Abp-WT, a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NX0_H Crystal structure of Abp-WT, a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus] |
| 4NXK_A | 4.06e-17 | 37 | 439 | 26 | 442 | Crystalstructure of Abp-D197A, a catalytic mutant of a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NXK_B Crystal structure of Abp-D197A, a catalytic mutant of a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NXK_C Crystal structure of Abp-D197A, a catalytic mutant of a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NXK_D Crystal structure of Abp-D197A, a catalytic mutant of a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NXK_E Crystal structure of Abp-D197A, a catalytic mutant of a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NXK_F Crystal structure of Abp-D197A, a catalytic mutant of a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NXK_G Crystal structure of Abp-D197A, a catalytic mutant of a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NXK_H Crystal structure of Abp-D197A, a catalytic mutant of a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4NZF_A Crystal structure of Abp-D197A (a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus), in complex with arabinose [Geobacillus stearothermophilus],4NZF_B Crystal structure of Abp-D197A (a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus), in complex with arabinose [Geobacillus stearothermophilus],4NZF_C Crystal structure of Abp-D197A (a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus), in complex with arabinose [Geobacillus stearothermophilus],4NZF_D Crystal structure of Abp-D197A (a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus), in complex with arabinose [Geobacillus stearothermophilus],4NZF_E Crystal structure of Abp-D197A (a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus), in complex with arabinose [Geobacillus stearothermophilus],4NZF_F Crystal structure of Abp-D197A (a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus), in complex with arabinose [Geobacillus stearothermophilus],4NZF_G Crystal structure of Abp-D197A (a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus), in complex with arabinose [Geobacillus stearothermophilus],4NZF_H Crystal structure of Abp-D197A (a GH27-b-L-arabinopyranosidase from Geobacillus stearothermophilus), in complex with arabinose [Geobacillus stearothermophilus] |
| 3CC1_A | 4.04e-11 | 37 | 440 | 13 | 432 | ChainA, Putative alpha-N-acetylgalactosaminidase [Halalkalibacterium halodurans C-125],3CC1_B Chain B, Putative alpha-N-acetylgalactosaminidase [Halalkalibacterium halodurans C-125] |
| 3A21_A | 1.95e-08 | 36 | 418 | 12 | 364 | CrystalStructure of Streptomyces avermitilis beta-L-Arabinopyranosidase [Streptomyces avermitilis],3A21_B Crystal Structure of Streptomyces avermitilis beta-L-Arabinopyranosidase [Streptomyces avermitilis],3A22_A Crystal Structure of beta-L-Arabinopyranosidase complexed with L-arabinose [Streptomyces avermitilis],3A22_B Crystal Structure of beta-L-Arabinopyranosidase complexed with L-arabinose [Streptomyces avermitilis],3A23_A Crystal Structure of beta-L-Arabinopyranosidase complexed with D-galactose [Streptomyces avermitilis],3A23_B Crystal Structure of beta-L-Arabinopyranosidase complexed with D-galactose [Streptomyces avermitilis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q44052 | 8.52e-92 | 26 | 475 | 53 | 529 | Isomalto-dextranase OS=Arthrobacter globiformis OX=1665 GN=imd PE=1 SV=1 |
| Q9FXT4 | 2.17e-06 | 34 | 440 | 61 | 417 | Alpha-galactosidase OS=Oryza sativa subsp. japonica OX=39947 GN=Os10g0493600 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
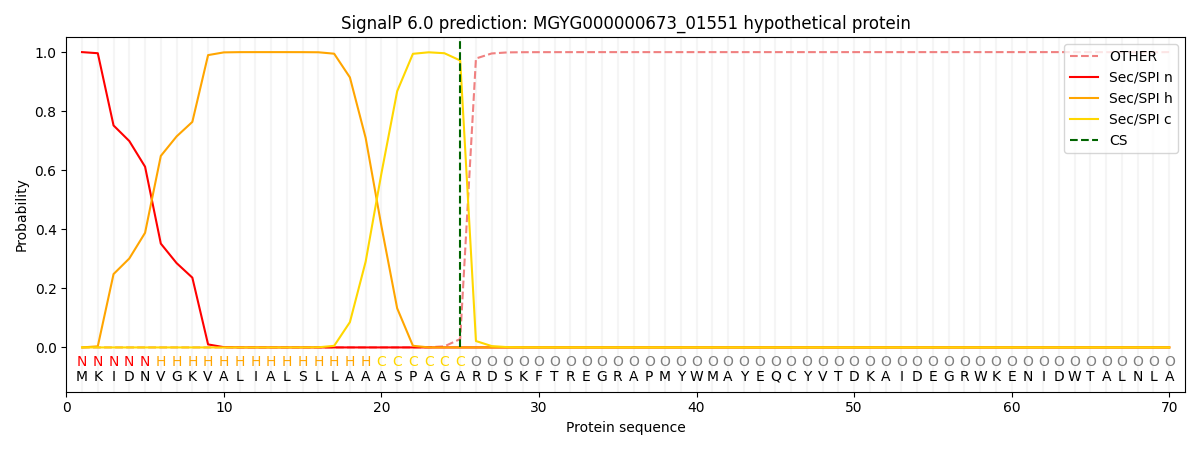
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000560 | 0.998509 | 0.000221 | 0.000258 | 0.000214 | 0.000192 |
