You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000000883_01495
You are here: Home > Sequence: MGYG000000883_01495
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | CAG-170 sp900555665 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Oscillospiraceae; CAG-170; CAG-170 sp900555665 | |||||||||||
| CAZyme ID | MGYG000000883_01495 | |||||||||||
| CAZy Family | CE4 | |||||||||||
| CAZyme Description | Peptidoglycan-N-acetylmuramic acid deacetylase PdaA | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 3850; End: 4659 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE4 | 69 | 196 | 9.3e-35 | 0.9461538461538461 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| TIGR02884 | spore_pdaA | 3.48e-110 | 42 | 263 | 2 | 223 | delta-lactam-biosynthetic de-N-acetylase. Muramic delta-lactam is an unusual constituent of peptidoglycan, found only in bacterial spores in the peptidoglycan wall, or spore cortex. The proteins in this family are PdaA (yfjS), a member of a larger family of polysaccharide deacetylases, and are specificially involved in delta-lactam biosynthesis. PdaA acts immediately after CwlD, an N-acetylmuramoyl-L-alanine amidase and performs a de-N-acetylation. PdaA may also perform the following transpeptidation for lactam ring formation, as heterologous expression in E. coli of CwlD and PdaA together is sufficient for delta-lactam production. [Cell envelope, Biosynthesis and degradation of murein sacculus and peptidoglycan, Cellular processes, Sporulation and germination] |
| cd10948 | CE4_BsPdaA_like | 2.96e-108 | 41 | 260 | 4 | 223 | Catalytic NodB homology domain of Bacillus subtilis polysaccharide deacetylase PdaA, and its bacterial homologs. The Bacillus subtilis genome contains six polysaccharide deacetylase gene homologs: pdaA, pdaB (previously known as ybaN), yheN, yjeA, yxkH and ylxY. This family is represented by Bacillus subtilis pdaA gene encoding polysaccharide deacetylase BsPdaA, which is a member of the carbohydrate esterase 4 (CE4) superfamily. BsPdaA deacetylates peptidoglycan N-acetylmuramic acid (MurNAc) residues to facilitate the formation of muramic delta-lactam, which is required for recognition of germination lytic enzymes. BsPdaA deficiency leads to the absence of muramic delta-lactam residues in the spore cortex. Like other CE4 esterases, BsPdaA consists of a single catalytic NodB homology domain that appears to adopt a deformed (beta/alpha)8 barrel fold with a putative substrate binding groove harboring the majority of the conserved residues. It utilizes a general acid/base catalytic mechanism involving a tetrahedral transition intermediate, where a water molecule functions as the nucleophile tightly associated to the zinc cofactor. |
| cd10917 | CE4_NodB_like_6s_7s | 1.19e-61 | 76 | 249 | 1 | 169 | Catalytic NodB homology domain of rhizobial NodB-like proteins. This family belongs to the large and functionally diverse carbohydrate esterase 4 (CE4) superfamily, whose members show strong sequence similarity with some variability due to their distinct carbohydrate substrates. It includes many rhizobial NodB chitooligosaccharide N-deacetylase (EC 3.5.1.-)-like proteins, mainly from bacteria and eukaryotes, such as chitin deacetylases (EC 3.5.1.41), bacterial peptidoglycan N-acetylglucosamine deacetylases (EC 3.5.1.-), and acetylxylan esterases (EC 3.1.1.72), which catalyze the N- or O-deacetylation of substrates such as acetylated chitin, peptidoglycan, and acetylated xylan. All members of this family contain a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold with 6- or 7 strands. Their catalytic activity is dependent on the presence of a divalent cation, preferably cobalt or zinc, and they employ a conserved His-His-Asp zinc-binding triad closely associated with the conserved catalytic base (aspartic acid) and acid (histidine) to carry out acid/base catalysis. Several family members show diversity both in metal ion specificities and in the residues that coordinate the metal. |
| TIGR02764 | spore_ybaN_pdaB | 3.97e-49 | 71 | 263 | 1 | 190 | polysaccharide deacetylase family sporulation protein PdaB. This model describes the YbaN protein family, also called PdaB and SpoVIE, of Gram-positive bacteria. Although ybaN null mutants have only a mild sporulation defect, ybaN/ytrI double mutants show drastically reducted sporulation efficiencies. This synthetic defect suggests the role of this sigmaE-controlled gene in sporulation had been masked by functional redundancy. Members of this family are homologous to a characterized polysaccharide deacetylase; the exact function this protein family is unknown. [Cellular processes, Sporulation and germination] |
| COG0726 | CDA1 | 7.12e-49 | 67 | 265 | 56 | 258 | Peptidoglycan/xylan/chitin deacetylase, PgdA/CDA1 family [Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BCI60049.1 | 3.89e-110 | 34 | 263 | 36 | 265 |
| QIB56171.1 | 9.22e-110 | 33 | 263 | 38 | 268 |
| QMW81094.1 | 9.22e-110 | 33 | 263 | 38 | 268 |
| ASU30505.1 | 6.13e-108 | 40 | 263 | 45 | 268 |
| ANU77704.1 | 6.13e-108 | 40 | 263 | 45 | 268 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1W1A_1 | 4.11e-66 | 34 | 263 | 15 | 246 | Structureof Bacillus subtilis PdaA in complex with NAG, a family 4 Carbohydrate esterase. [Bacillus subtilis],1W1A_2 Structure of Bacillus subtilis PdaA in complex with NAG, a family 4 Carbohydrate esterase. [Bacillus subtilis],1W1B_1 Structure of Bacillus subtilis PdaA with Cadmium, a family 4 Carbohydrate esterase. [Bacillus subtilis],1W1B_2 Structure of Bacillus subtilis PdaA with Cadmium, a family 4 Carbohydrate esterase. [Bacillus subtilis] |
| 1W17_A | 4.94e-66 | 34 | 263 | 21 | 252 | Structureof Bacillus subtilis PdaA, a family 4 Carbohydrate esterase. [Bacillus subtilis],1W17_B Structure of Bacillus subtilis PdaA, a family 4 Carbohydrate esterase. [Bacillus subtilis] |
| 1NY1_A | 1.11e-64 | 41 | 263 | 7 | 229 | CrystalStructure Of B. Subtilis Polysaccharide Deacetylase Northeast Structural Genomics Consortium Target Sr127. [Bacillus subtilis],1NY1_B Crystal Structure Of B. Subtilis Polysaccharide Deacetylase Northeast Structural Genomics Consortium Target Sr127. [Bacillus subtilis] |
| 2J13_A | 7.32e-59 | 41 | 266 | 19 | 244 | Structureof a family 4 carbohydrate esterase from Bacillus anthracis [Bacillus anthracis str. Ames] |
| 6HM9_A | 1.10e-30 | 69 | 263 | 78 | 266 | Crystalstructure of a BA3943 mutant,a CE4 family pseudoenzyme with restored enzymatic activity. [Bacillus anthracis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q04729 | 6.89e-69 | 42 | 263 | 32 | 253 | Uncharacterized 30.6 kDa protein in fumA 3'region OS=Geobacillus stearothermophilus OX=1422 PE=3 SV=1 |
| O34928 | 2.70e-65 | 34 | 263 | 21 | 252 | Peptidoglycan-N-acetylmuramic acid deacetylase PdaA OS=Bacillus subtilis (strain 168) OX=224308 GN=pdaA PE=1 SV=1 |
| A0A3Q0NBH7 | 2.27e-30 | 76 | 264 | 266 | 446 | Peptidoglycan-N-acetylglucosamine deacetylase PgdA OS=Listeria monocytogenes serotype 1/2a (strain EGD / Mackaness) OX=1334565 GN=pgdA PE=1 SV=1 |
| Q8Y9V5 | 2.27e-30 | 76 | 264 | 266 | 446 | Peptidoglycan-N-acetylglucosamine deacetylase PgdA OS=Listeria monocytogenes serovar 1/2a (strain ATCC BAA-679 / EGD-e) OX=169963 GN=pgdA PE=1 SV=1 |
| A0A0H3GDH9 | 2.27e-30 | 76 | 264 | 266 | 446 | Peptidoglycan-N-acetylglucosamine deacetylase PgdA OS=Listeria monocytogenes serotype 1/2a (strain 10403S) OX=393133 GN=pgdA PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
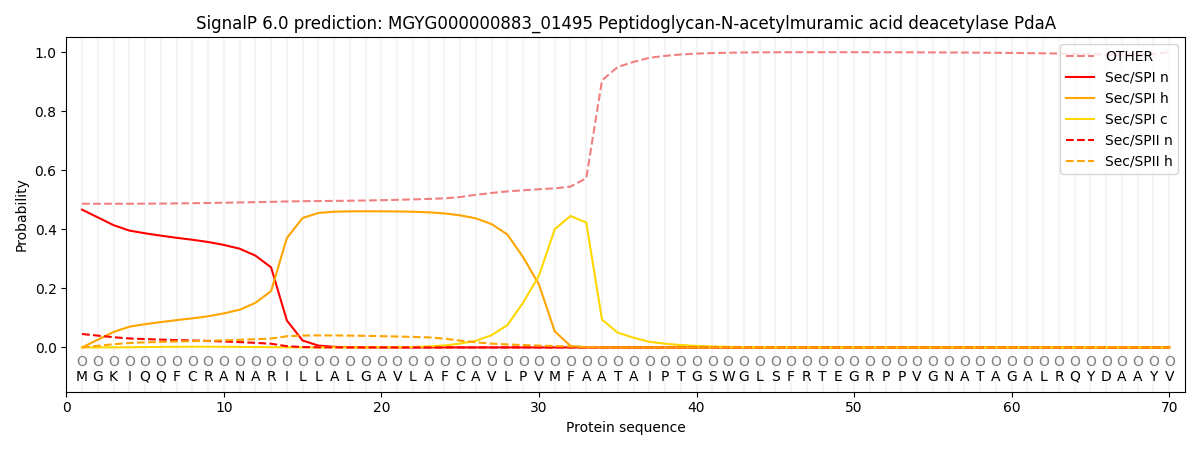
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.501023 | 0.443831 | 0.048548 | 0.003416 | 0.001157 | 0.002025 |

