You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001000_00080
You are here: Home > Sequence: MGYG000001000_00080
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
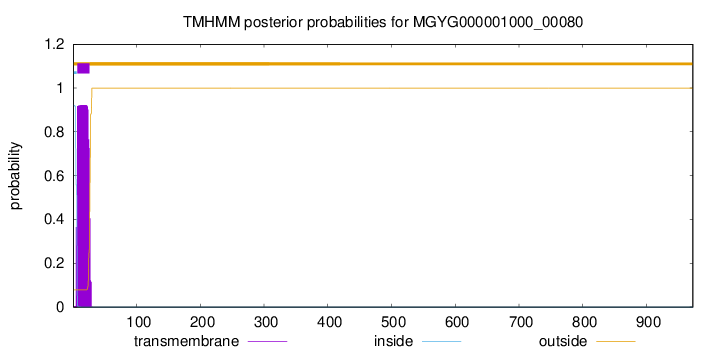
TMHMM annotations
Basic Information help
| Species | UBA4716 sp900556575 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Monoglobales_A; UBA1381; UBA4716; UBA4716 sp900556575 | |||||||||||
| CAZyme ID | MGYG000001000_00080 | |||||||||||
| CAZy Family | PL11 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 20959; End: 23880 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL11 | 59 | 649 | 1.5e-236 | 0.9965694682675815 |
| CBM32 | 852 | 968 | 1.7e-20 | 0.9032258064516129 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd10318 | RGL11 | 0.0 | 61 | 634 | 1 | 564 | Rhamnogalacturonan lyase of the polysaccharide lyase family 11. The rhamnogalacturonan lyase of the polysaccharide lyase family 11 (RGL11) cleaves glycoside bonds in polygalacturonan as well as RG (rhamnogalacturonan) type-I through a beta-elimination reaction. Functionally characterized members of this family, YesW and YesX from Bacillus subtilis, cleave glycoside bonds between rhamnose and galacturonic acid residues in the RG-I region of plant cell wall pectin. YesW and YesX work synergistically, with YesW cleaving the glycoside bond of the RG chain endolytically, and YesX converting the resultant oligosaccharides through an exotype reaction. This domain is sometimes found in architectures with non-catalytic carbohydrate-binding modules (CBMs). There are two types of RG lyases, which both cleave the alpha-1,4 bonds of the RG-I main chain through a beta-elimination reaction, but belong to two structurally unrelated polysaccharide lyase (PL) families, 4 and 11. |
| pfam07833 | Cu_amine_oxidN1 | 7.42e-34 | 710 | 796 | 1 | 87 | Copper amine oxidase N-terminal domain. Copper amine oxidases catalyze the oxidative deamination of primary amines to the corresponding aldehydes, while reducing molecular oxygen to hydrogen peroxide. These enzymes are dimers of identical subunits, each comprising four domains. The N-terminal domain, which is absent in some amine oxidases, consists of a five-stranded antiparallel beta sheet twisted around an alpha helix. The D1 domains from the two subunits comprise the 'stalk' of the mushroom-shaped dimer, and interact with each other but do not pack tightly against each other. |
| pfam18370 | RGI_lyase | 5.23e-31 | 59 | 141 | 1 | 84 | Rhamnogalacturonan I lyases beta-sheet domain. This is the beta-sheet domain found in rhamnogalacturonan (RG) lyases, which are responsible for an initial cleavage of the RG type I (RG-I) region of plant cell wall pectin. Polysaccharide lyase family 11 carrying this domain, such as YesW (EC:4.2.2.23) and YesX (EC:4.2.2.24), cleave glycoside bonds between rhamnose and galacturonic acid residues in RG-I through a beta-elimination reaction. Other family members carrying this domain are hemagglutinin A, lysine gingipain (Kgp) and Chitinase C (EC:3.2.1.14). |
| pfam00754 | F5_F8_type_C | 7.11e-17 | 849 | 968 | 1 | 127 | F5/8 type C domain. This domain is also known as the discoidin (DS) domain family. |
| pfam07833 | Cu_amine_oxidN1 | 3.79e-12 | 674 | 740 | 33 | 93 | Copper amine oxidase N-terminal domain. Copper amine oxidases catalyze the oxidative deamination of primary amines to the corresponding aldehydes, while reducing molecular oxygen to hydrogen peroxide. These enzymes are dimers of identical subunits, each comprising four domains. The N-terminal domain, which is absent in some amine oxidases, consists of a five-stranded antiparallel beta sheet twisted around an alpha helix. The D1 domains from the two subunits comprise the 'stalk' of the mushroom-shaped dimer, and interact with each other but do not pack tightly against each other. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AGE62575.1 | 3.23e-213 | 59 | 649 | 15 | 593 |
| AKD34106.1 | 6.24e-213 | 59 | 649 | 34 | 612 |
| ASK22710.1 | 6.24e-213 | 59 | 649 | 34 | 612 |
| ARV97666.1 | 6.24e-213 | 59 | 649 | 34 | 612 |
| AGI27974.1 | 6.24e-213 | 59 | 649 | 34 | 612 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2Z8R_A | 1.64e-213 | 59 | 649 | 3 | 581 | Crystalstructure of rhamnogalacturonan lyase YesW at 1.40 A resolution [Bacillus subtilis],2Z8R_B Crystal structure of rhamnogalacturonan lyase YesW at 1.40 A resolution [Bacillus subtilis],2Z8S_A Crystal structure of rhamnogalacturonan lyase YesW complexed with digalacturonic acid [Bacillus subtilis],2Z8S_B Crystal structure of rhamnogalacturonan lyase YesW complexed with digalacturonic acid [Bacillus subtilis],2ZUX_A Crystal structure of rhamnogalacturonan lyase YesW complexed with rhamnose [Bacillus subtilis],2ZUX_B Crystal structure of rhamnogalacturonan lyase YesW complexed with rhamnose [Bacillus subtilis] |
| 4CAG_A | 1.14e-203 | 59 | 649 | 9 | 588 | Bacilluslicheniformis Rhamnogalacturonan Lyase PL11 [Bacillus licheniformis] |
| 2ZUY_A | 2.32e-195 | 58 | 650 | 5 | 603 | Crystalstructure of exotype rhamnogalacturonan lyase YesX [Bacillus subtilis] |
| 5ZU6_A | 2.78e-21 | 851 | 956 | 38 | 144 | ACBM32 derived from alginate lyase B (AlyB-OU02) [Vibrio] |
| 5ZU5_A | 4.43e-19 | 851 | 956 | 38 | 144 | Crystalstructure of a full length alginate lyase with CBM domain [Vibrio splendidus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| O31526 | 2.45e-212 | 59 | 649 | 40 | 618 | Rhamnogalacturonan endolyase YesW OS=Bacillus subtilis (strain 168) OX=224308 GN=yesW PE=1 SV=1 |
| O31527 | 9.71e-195 | 58 | 650 | 5 | 603 | Rhamnogalacturonan exolyase YesX OS=Bacillus subtilis (strain 168) OX=224308 GN=yesX PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
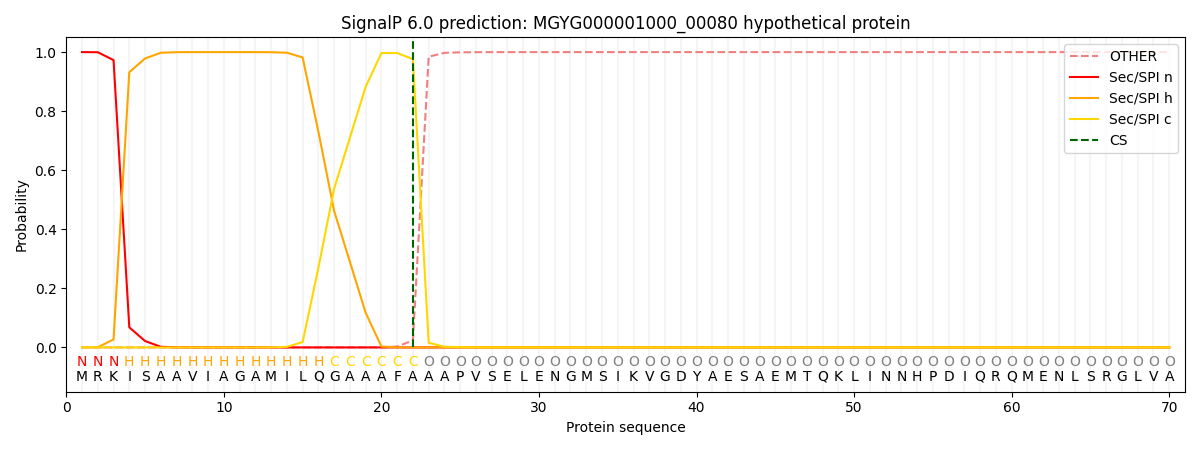
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000247 | 0.999028 | 0.000161 | 0.000184 | 0.000178 | 0.000152 |