You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001000_00156
You are here: Home > Sequence: MGYG000001000_00156
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | UBA4716 sp900556575 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Monoglobales_A; UBA1381; UBA4716; UBA4716 sp900556575 | |||||||||||
| CAZyme ID | MGYG000001000_00156 | |||||||||||
| CAZy Family | CE4 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 12604; End: 13479 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE4 | 91 | 217 | 1e-30 | 0.9538461538461539 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd10948 | CE4_BsPdaA_like | 7.69e-102 | 60 | 280 | 1 | 223 | Catalytic NodB homology domain of Bacillus subtilis polysaccharide deacetylase PdaA, and its bacterial homologs. The Bacillus subtilis genome contains six polysaccharide deacetylase gene homologs: pdaA, pdaB (previously known as ybaN), yheN, yjeA, yxkH and ylxY. This family is represented by Bacillus subtilis pdaA gene encoding polysaccharide deacetylase BsPdaA, which is a member of the carbohydrate esterase 4 (CE4) superfamily. BsPdaA deacetylates peptidoglycan N-acetylmuramic acid (MurNAc) residues to facilitate the formation of muramic delta-lactam, which is required for recognition of germination lytic enzymes. BsPdaA deficiency leads to the absence of muramic delta-lactam residues in the spore cortex. Like other CE4 esterases, BsPdaA consists of a single catalytic NodB homology domain that appears to adopt a deformed (beta/alpha)8 barrel fold with a putative substrate binding groove harboring the majority of the conserved residues. It utilizes a general acid/base catalytic mechanism involving a tetrahedral transition intermediate, where a water molecule functions as the nucleophile tightly associated to the zinc cofactor. |
| TIGR02884 | spore_pdaA | 1.52e-99 | 63 | 282 | 1 | 222 | delta-lactam-biosynthetic de-N-acetylase. Muramic delta-lactam is an unusual constituent of peptidoglycan, found only in bacterial spores in the peptidoglycan wall, or spore cortex. The proteins in this family are PdaA (yfjS), a member of a larger family of polysaccharide deacetylases, and are specificially involved in delta-lactam biosynthesis. PdaA acts immediately after CwlD, an N-acetylmuramoyl-L-alanine amidase and performs a de-N-acetylation. PdaA may also perform the following transpeptidation for lactam ring formation, as heterologous expression in E. coli of CwlD and PdaA together is sufficient for delta-lactam production. [Cell envelope, Biosynthesis and degradation of murein sacculus and peptidoglycan, Cellular processes, Sporulation and germination] |
| cd10917 | CE4_NodB_like_6s_7s | 1.18e-55 | 98 | 271 | 1 | 171 | Catalytic NodB homology domain of rhizobial NodB-like proteins. This family belongs to the large and functionally diverse carbohydrate esterase 4 (CE4) superfamily, whose members show strong sequence similarity with some variability due to their distinct carbohydrate substrates. It includes many rhizobial NodB chitooligosaccharide N-deacetylase (EC 3.5.1.-)-like proteins, mainly from bacteria and eukaryotes, such as chitin deacetylases (EC 3.5.1.41), bacterial peptidoglycan N-acetylglucosamine deacetylases (EC 3.5.1.-), and acetylxylan esterases (EC 3.1.1.72), which catalyze the N- or O-deacetylation of substrates such as acetylated chitin, peptidoglycan, and acetylated xylan. All members of this family contain a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold with 6- or 7 strands. Their catalytic activity is dependent on the presence of a divalent cation, preferably cobalt or zinc, and they employ a conserved His-His-Asp zinc-binding triad closely associated with the conserved catalytic base (aspartic acid) and acid (histidine) to carry out acid/base catalysis. Several family members show diversity both in metal ion specificities and in the residues that coordinate the metal. |
| COG0726 | CDA1 | 9.69e-43 | 81 | 284 | 48 | 257 | Peptidoglycan/xylan/chitin deacetylase, PgdA/CDA1 family [Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
| cd10959 | CE4_NodB_like_3 | 1.18e-41 | 100 | 280 | 3 | 187 | Catalytic NodB homology domain of uncharacterized bacterial polysaccharide deacetylases. This family includes many uncharacterized bacterial polysaccharide deacetylases. Although their biological function still remains unknown, members in this family show high sequence homology to the catalytic NodB homology domain of Streptococcus pneumoniae polysaccharide deacetylase PgdA (SpPgdA), which is an extracellular metal-dependent polysaccharide deacetylase with de-N-acetylase activity toward a hexamer of chitooligosaccharide N-acetylglucosamine, but not shorter chitooligosaccharides or a synthetic peptidoglycan tetrasaccharide. Like SpPgdA, this family is a member of the carbohydrate esterase 4 (CE4) superfamily. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BAB04614.1 | 1.18e-79 | 55 | 283 | 25 | 255 |
| ADC50191.1 | 3.04e-75 | 58 | 283 | 27 | 254 |
| ANY68957.1 | 1.19e-73 | 56 | 282 | 25 | 255 |
| QFK73175.1 | 3.27e-73 | 55 | 286 | 12 | 245 |
| QNO14590.1 | 5.96e-73 | 58 | 282 | 28 | 254 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 2J13_A | 1.11e-66 | 64 | 282 | 20 | 240 | Structureof a family 4 carbohydrate esterase from Bacillus anthracis [Bacillus anthracis str. Ames] |
| 1W1A_1 | 1.10e-63 | 55 | 282 | 16 | 245 | Structureof Bacillus subtilis PdaA in complex with NAG, a family 4 Carbohydrate esterase. [Bacillus subtilis],1W1A_2 Structure of Bacillus subtilis PdaA in complex with NAG, a family 4 Carbohydrate esterase. [Bacillus subtilis],1W1B_1 Structure of Bacillus subtilis PdaA with Cadmium, a family 4 Carbohydrate esterase. [Bacillus subtilis],1W1B_2 Structure of Bacillus subtilis PdaA with Cadmium, a family 4 Carbohydrate esterase. [Bacillus subtilis] |
| 1W17_A | 1.32e-63 | 55 | 282 | 22 | 251 | Structureof Bacillus subtilis PdaA, a family 4 Carbohydrate esterase. [Bacillus subtilis],1W17_B Structure of Bacillus subtilis PdaA, a family 4 Carbohydrate esterase. [Bacillus subtilis] |
| 1NY1_A | 5.94e-62 | 62 | 282 | 6 | 228 | CrystalStructure Of B. Subtilis Polysaccharide Deacetylase Northeast Structural Genomics Consortium Target Sr127. [Bacillus subtilis],1NY1_B Crystal Structure Of B. Subtilis Polysaccharide Deacetylase Northeast Structural Genomics Consortium Target Sr127. [Bacillus subtilis] |
| 6HM9_A | 1.07e-27 | 77 | 282 | 65 | 265 | Crystalstructure of a BA3943 mutant,a CE4 family pseudoenzyme with restored enzymatic activity. [Bacillus anthracis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| O34928 | 7.25e-63 | 55 | 282 | 22 | 251 | Peptidoglycan-N-acetylmuramic acid deacetylase PdaA OS=Bacillus subtilis (strain 168) OX=224308 GN=pdaA PE=1 SV=1 |
| Q04729 | 2.18e-62 | 62 | 282 | 30 | 252 | Uncharacterized 30.6 kDa protein in fumA 3'region OS=Geobacillus stearothermophilus OX=1422 PE=3 SV=1 |
| Q52845 | 1.75e-31 | 93 | 284 | 16 | 219 | Chitooligosaccharide deacetylase OS=Mesorhizobium japonicum (strain LMG 29417 / CECT 9101 / MAFF 303099) OX=266835 GN=nodB PE=3 SV=2 |
| P04675 | 3.10e-27 | 91 | 282 | 14 | 217 | Chitooligosaccharide deacetylase OS=Bradyrhizobium sp. (strain ANU 289) OX=186901 GN=nodB PE=3 SV=2 |
| Q8Y9V5 | 2.23e-26 | 98 | 286 | 266 | 448 | Peptidoglycan-N-acetylglucosamine deacetylase PgdA OS=Listeria monocytogenes serovar 1/2a (strain ATCC BAA-679 / EGD-e) OX=169963 GN=pgdA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
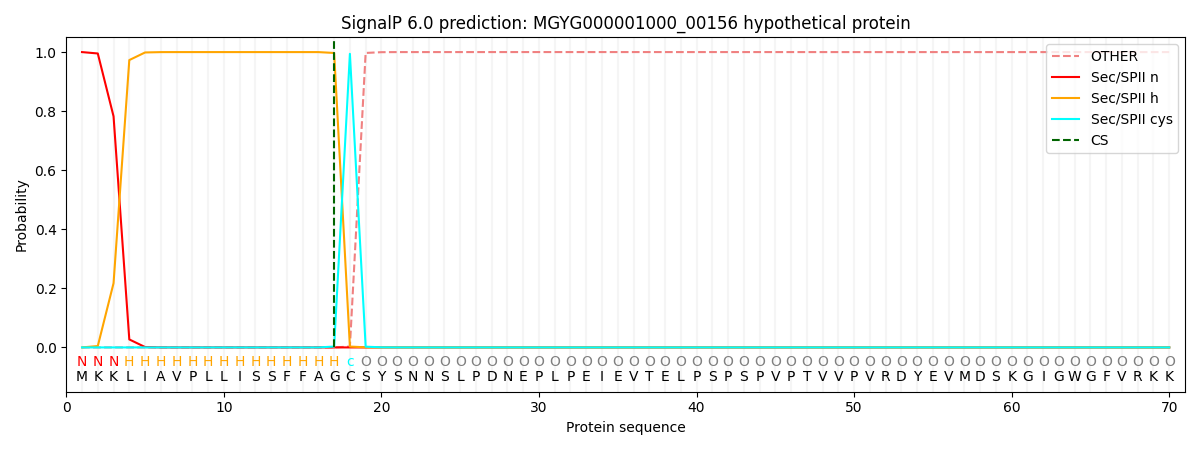
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000000 | 0.000000 | 1.000069 | 0.000000 | 0.000000 | 0.000000 |
