You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001042_00410
You are here: Home > Sequence: MGYG000001042_00410
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Prevotella sp000436595 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotella; Prevotella sp000436595 | |||||||||||
| CAZyme ID | MGYG000001042_00410 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 71409; End: 72677 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL1 | 79 | 290 | 5.9e-44 | 0.9108910891089109 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3866 | PelB | 7.51e-38 | 16 | 359 | 18 | 342 | Pectate lyase [Carbohydrate transport and metabolism]. |
| smart00656 | Amb_all | 5.46e-23 | 90 | 291 | 4 | 187 | Amb_all domain. |
| pfam00544 | Pec_lyase_C | 1.03e-11 | 98 | 290 | 30 | 211 | Pectate lyase. This enzyme forms a right handed beta helix structure. Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QYR11586.1 | 1.04e-90 | 19 | 364 | 35 | 362 |
| QQQ30328.1 | 5.32e-84 | 55 | 361 | 69 | 364 |
| AZA73090.1 | 5.32e-84 | 55 | 361 | 69 | 364 |
| AZA61254.1 | 8.40e-83 | 55 | 361 | 69 | 364 |
| AZA64152.1 | 3.34e-82 | 55 | 360 | 69 | 363 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3ZSC_A | 4.33e-17 | 38 | 267 | 7 | 214 | Catalyticfunction and substrate recognition of the pectate lyase from Thermotoga maritima [Thermotoga maritima] |
| 1VBL_A | 3.60e-13 | 95 | 306 | 125 | 353 | Structureof the thermostable pectate lyase PL 47 [Bacillus sp. TS-47] |
| 5AMV_A | 5.98e-13 | 136 | 288 | 151 | 323 | Structuralinsights into the loss of catalytic competence in pectate lyase at low pH [Bacillus subtilis],5X2I_A Polygalacturonate Lyase by Fusing with a Self-assembling Amphipathic Peptide [Bacillus subtilis subsp. subtilis str. 168] |
| 1BN8_A | 6.50e-13 | 136 | 288 | 172 | 344 | BacillusSubtilis Pectate Lyase [Bacillus subtilis] |
| 3KRG_A | 7.98e-13 | 136 | 288 | 151 | 323 | ChainA, Pectate lyase [Bacillus subtilis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| B1L969 | 7.60e-21 | 38 | 267 | 32 | 239 | Pectate trisaccharide-lyase OS=Thermotoga sp. (strain RQ2) OX=126740 GN=pelA PE=3 SV=1 |
| Q9WYR4 | 1.95e-20 | 38 | 267 | 34 | 241 | Pectate trisaccharide-lyase OS=Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8) OX=243274 GN=pelA PE=1 SV=1 |
| Q0CBV0 | 1.16e-18 | 20 | 360 | 27 | 317 | Probable pectate lyase B OS=Aspergillus terreus (strain NIH 2624 / FGSC A1156) OX=341663 GN=plyB PE=3 SV=1 |
| B8NBC2 | 1.12e-16 | 55 | 366 | 48 | 324 | Probable pectate lyase B OS=Aspergillus flavus (strain ATCC 200026 / FGSC A1120 / IAM 13836 / NRRL 3357 / JCM 12722 / SRRC 167) OX=332952 GN=plyB PE=3 SV=1 |
| Q2TZY0 | 1.12e-16 | 55 | 366 | 48 | 324 | Probable pectate lyase B OS=Aspergillus oryzae (strain ATCC 42149 / RIB 40) OX=510516 GN=plyB PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
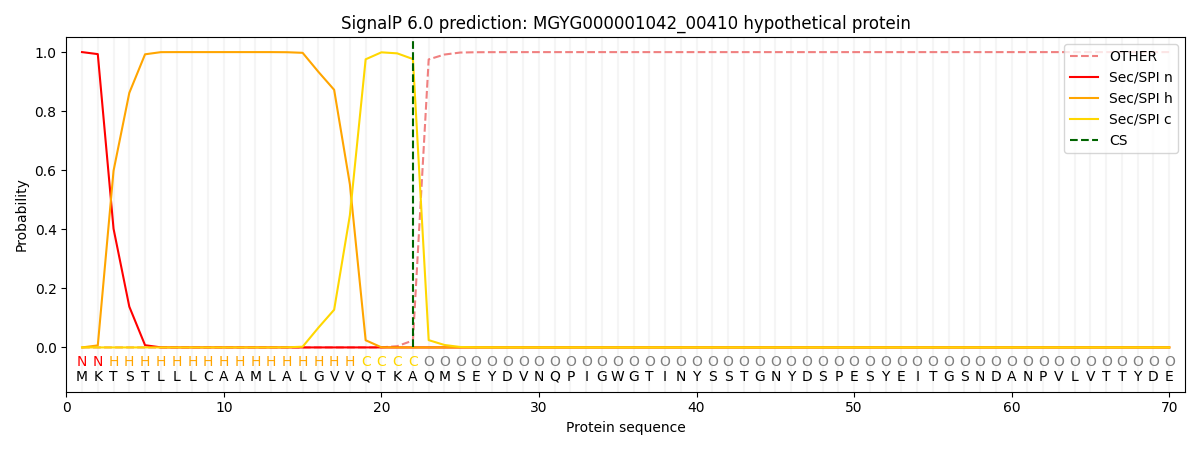
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000206 | 0.999144 | 0.000165 | 0.000165 | 0.000152 | 0.000143 |
