You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001056_02383
You are here: Home > Sequence: MGYG000001056_02383
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Prevotella sp900556395 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Prevotella; Prevotella sp900556395 | |||||||||||
| CAZyme ID | MGYG000001056_02383 | |||||||||||
| CAZy Family | GH13 | |||||||||||
| CAZyme Description | Pullulanase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 67826; End: 69796 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH13 | 174 | 507 | 1e-110 | 0.9965397923875432 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd11341 | AmyAc_Pullulanase_LD-like | 0.0 | 140 | 550 | 2 | 406 | Alpha amylase catalytic domain found in Pullulanase (also called dextrinase; alpha-dextrin endo-1,6-alpha glucosidase), limit dextrinase, and related proteins. Pullulanase is an enzyme with action similar to that of isoamylase; it cleaves 1,6-alpha-glucosidic linkages in pullulan, amylopectin, and glycogen, and in alpha-and beta-amylase limit-dextrins of amylopectin and glycogen. Pullulanases are very similar to limit dextrinases, although they differ in their action on glycogen and the rate of hydrolysis of limit dextrins. The Alpha-amylase family comprises the largest family of glycoside hydrolases (GH), with the majority of enzymes acting on starch, glycogen, and related oligo- and polysaccharides. These proteins catalyze the transformation of alpha-1,4 and alpha-1,6 glucosidic linkages with retention of the anomeric center. The protein is described as having 3 domains: A, B, C. A is a (beta/alpha) 8-barrel; B is a loop between the beta 3 strand and alpha 3 helix of A; C is the C-terminal extension characterized by a Greek key. The majority of the enzymes have an active site cleft found between domains A and B where a triad of catalytic residues (Asp, Glu and Asp) performs catalysis. Other members of this family have lost the catalytic activity as in the case of the human 4F2hc, or only have 2 residues that serve as the catalytic nucleophile and the acid/base, such as Thermus A4 beta-galactosidase with 2 Glu residues (GH42) and human alpha-galactosidase with 2 Asp residues (GH31). The family members are quite extensive and include: alpha amylase, maltosyltransferase, cyclodextrin glycotransferase, maltogenic amylase, neopullulanase, isoamylase, 1,4-alpha-D-glucan maltotetrahydrolase, 4-alpha-glucotransferase, oligo-1,6-glucosidase, amylosucrase, sucrose phosphorylase, and amylomaltase. |
| TIGR02104 | pulA_typeI | 0.0 | 28 | 633 | 13 | 605 | pullulanase, type I. Pullulan is an unusual, industrially important polysaccharide in which short alpha-1,4 chains (maltotriose) are connected in alpha-1,6 linkages. Enzymes that cleave alpha-1,6 linkages in pullulan and release maltotriose are called pullulanases although pullulan itself may not be the natural substrate. This family consists of pullulanases related to the subfamilies described in TIGR02102 and TIGR02103 but having a different domain architecture with shorter sequences. Members are called type I pullulanases. |
| COG1523 | PulA | 2.99e-136 | 37 | 654 | 70 | 690 | Pullulanase/glycogen debranching enzyme [Carbohydrate transport and metabolism]. |
| TIGR02103 | pullul_strch | 1.63e-115 | 34 | 612 | 135 | 848 | alpha-1,6-glucosidases, pullulanase-type. Members of this protein family include secreted (or membrane-anchored) pullulanases of Gram-negative bacteria and pullulanase-type starch debranching enzymes of plants. Both enzymes hydrolyze alpha-1,6 glycosidic linkages. Pullulan is an unusual, industrially important polysaccharide in which short alpha-1,4 chains (maltotriose) are connected in alpha-1,6 linkages. Enzymes that cleave alpha-1,6 linkages in pullulan and release maltotriose are called pullulanases although pullulan itself may not be the natural substrate. This family is closely homologous to, but architecturally different from, the Gram-positive pullulanases of Gram-positive bacteria (TIGR02102). [Energy metabolism, Biosynthesis and degradation of polysaccharides] |
| PLN02877 | PLN02877 | 3.60e-103 | 31 | 616 | 219 | 923 | alpha-amylase/limit dextrinase |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QNT66905.1 | 0.0 | 1 | 656 | 1 | 656 |
| AGB28439.1 | 0.0 | 26 | 655 | 34 | 660 |
| ALO49594.1 | 0.0 | 1 | 655 | 1 | 640 |
| BCS85624.1 | 0.0 | 22 | 656 | 14 | 628 |
| VEH15006.1 | 4.75e-316 | 10 | 654 | 6 | 628 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6JEQ_A | 5.09e-160 | 30 | 616 | 44 | 618 | Crystalstructure of Pullulanase from Paenibacillus barengoltzii complex with beta-cyclodextrin [Paenibacillus barengoltzii],6JFJ_A Crystal structure of Pullulanase from Paenibacillus barengoltzii complex with maltohexaose and alpha-cyclodextrin [Paenibacillus barengoltzii],6JFX_A Crystal structure of Pullulanase from Paenibacillus barengoltzii complex with maltopentaose [Paenibacillus barengoltzii],6JHF_A Crystal structure of apo Pullulanase from Paenibacillus barengoltzii [Paenibacillus barengoltzii],6JHG_A Crystal structure of apo Pullulanase from Paenibacillus barengoltzii in space group P212121 [Paenibacillus barengoltzii] |
| 6JHI_A | 8.08e-159 | 30 | 616 | 44 | 618 | Crystalstructure of mutant D470A of Pullulanase from Paenibacillus barengoltzii complexed with maltotetraose [Paenibacillus barengoltzii] |
| 6JHH_A | 8.08e-159 | 30 | 616 | 44 | 618 | Crystalstructure of mutant D350A of Pullulanase from Paenibacillus barengoltzii complexed with maltotriose [Paenibacillus barengoltzii] |
| 2WAN_A | 2.72e-158 | 30 | 655 | 321 | 921 | Pullulanasefrom Bacillus acidopullulyticus [Bacillus acidopullulyticus] |
| 7LSA_A | 3.13e-128 | 31 | 656 | 68 | 714 | ChainA, Pullulanase [Ruminococcus bromii] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| O33840 | 3.75e-147 | 30 | 656 | 228 | 843 | Pullulanase OS=Thermotoga maritima (strain ATCC 43589 / DSM 3109 / JCM 10099 / NBRC 100826 / MSB8) OX=243274 GN=pulA PE=1 SV=2 |
| C0SPA0 | 4.95e-126 | 30 | 624 | 109 | 682 | Pullulanase OS=Bacillus subtilis (strain 168) OX=224308 GN=amyX PE=1 SV=1 |
| A0A0H2ZL64 | 2.46e-63 | 42 | 635 | 453 | 1096 | Pullulanase A OS=Streptococcus pneumoniae serotype 2 (strain D39 / NCTC 7466) OX=373153 GN=spuA PE=3 SV=1 |
| Q9F930 | 2.59e-63 | 42 | 635 | 475 | 1118 | Pullulanase A OS=Streptococcus pneumoniae OX=1313 GN=spuA PE=1 SV=1 |
| A0A0H2UNG0 | 4.68e-63 | 42 | 635 | 468 | 1111 | Pullulanase A OS=Streptococcus pneumoniae serotype 4 (strain ATCC BAA-334 / TIGR4) OX=170187 GN=spuA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
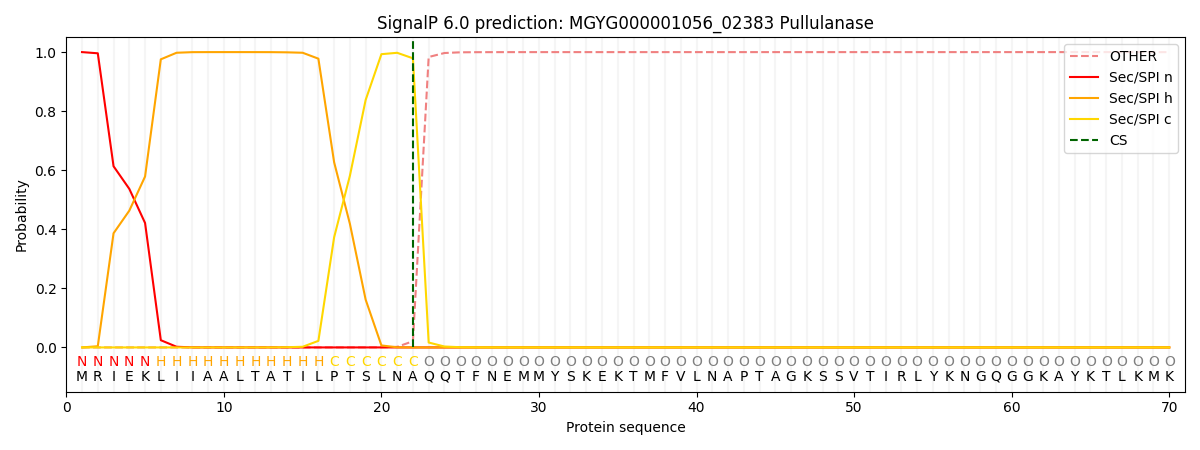
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000306 | 0.998964 | 0.000211 | 0.000187 | 0.000168 | 0.000151 |
