You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001073_01535
You are here: Home > Sequence: MGYG000001073_01535
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
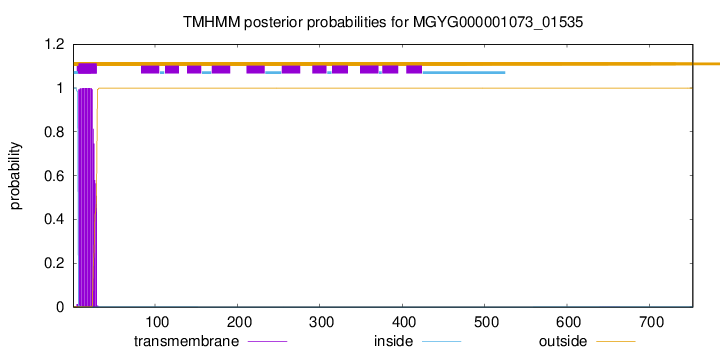
TMHMM annotations
Basic Information help
| Species | Fusobacterium_B sp900554885 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Fusobacteriota; Fusobacteriia; Fusobacteriales; Fusobacteriaceae; Fusobacterium_B; Fusobacterium_B sp900554885 | |||||||||||
| CAZyme ID | MGYG000001073_01535 | |||||||||||
| CAZy Family | GT51 | |||||||||||
| CAZyme Description | Penicillin-binding protein 1C | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 4639; End: 6900 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GT51 | 59 | 222 | 2.7e-48 | 0.9322033898305084 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG4953 | PbpC | 0.0 | 36 | 753 | 32 | 733 | Membrane carboxypeptidase/penicillin-binding protein PbpC [Cell wall/membrane/envelope biogenesis]. |
| TIGR02073 | PBP_1c | 0.0 | 35 | 751 | 6 | 726 | penicillin-binding protein 1C. This subfamily of the penicillin binding proteins includes the member from E. coli designated penicillin-binding protein 1C. Members have both transglycosylase and transpeptidase domains and are involved in forming cross-links in the late stages of peptidoglycan biosynthesis. All members of this subfamily are presumed to have the same basic function. [Cell envelope, Biosynthesis and degradation of murein sacculus and peptidoglycan] |
| COG0744 | MrcB | 1.31e-101 | 7 | 587 | 18 | 623 | Membrane carboxypeptidase (penicillin-binding protein) [Cell wall/membrane/envelope biogenesis]. |
| PRK11240 | PRK11240 | 1.29e-92 | 65 | 537 | 66 | 535 | penicillin-binding protein 1C; Provisional |
| TIGR02074 | PBP_1a_fam | 2.97e-87 | 65 | 538 | 6 | 501 | penicillin-binding protein, 1A family. Bacterial that synthesize a cell wall of peptidoglycan (murein) generally have several transglycosylases and transpeptidases for the task. This family consists of bifunctional transglycosylase/transpeptidase penicillin-binding proteins (PBP). In the Proteobacteria, this family includes PBP 1A but not the paralogous PBP 1B (TIGR02071). This family also includes related proteins, often designated PBP 1A, from other bacterial lineages. [Cell envelope, Biosynthesis and degradation of murein sacculus and peptidoglycan] |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AVQ31509.1 | 0.0 | 1 | 753 | 1 | 752 |
| VEH39720.1 | 0.0 | 1 | 753 | 1 | 752 |
| SQJ06920.1 | 0.0 | 1 | 753 | 1 | 752 |
| AVQ26788.1 | 0.0 | 1 | 753 | 1 | 752 |
| BBA50599.1 | 0.0 | 1 | 753 | 1 | 752 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5FGZ_A | 4.33e-36 | 70 | 557 | 165 | 683 | E.coli PBP1b in complex with FPI-1465 [Escherichia coli K-12],5HL9_A E. coli PBP1b in complex with acyl-ampicillin and moenomycin [Escherichia coli K-12],5HLA_A E. coli PBP1b in complex with acyl-cephalexin and moenomycin [Escherichia coli K-12],5HLB_A E. coli PBP1b in complex with acyl-aztreonam and moenomycin [Escherichia coli K-12],5HLD_A E. coli PBP1b in complex with acyl-CENTA and moenomycin [Escherichia coli K-12],6YN0_A Structure of E. coli PBP1b with a FtsN peptide activating transglycosylase activity [Escherichia coli K-12],7LQ6_A Chain A, Penicillin-binding protein 1B [Escherichia coli K-12] |
| 3VMA_A | 4.74e-36 | 70 | 557 | 186 | 704 | CrystalStructure of the Full-Length Transglycosylase PBP1b from Escherichia coli [Escherichia coli K-12] |
| 4OON_A | 6.19e-32 | 68 | 537 | 46 | 694 | Crystalstructure of PBP1a in complex with compound 17 ((4Z,8S,11E,14S)-5-(2-amino-1,3-thiazol-4-yl)-14-(5,6-dihydroxy-1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)-8-formyl-2-methyl-6-oxo-3,10-dioxa-4,7,11-triazatetradeca-4,11-diene-2,12,14-tricarboxylic acid) [Pseudomonas aeruginosa PAO1] |
| 3DWK_A | 5.20e-31 | 36 | 537 | 3 | 556 | ChainA, Penicillin-binding protein 2 [Staphylococcus aureus subsp. aureus COL],3DWK_B Chain B, Penicillin-binding protein 2 [Staphylococcus aureus subsp. aureus COL],3DWK_C Chain C, Penicillin-binding protein 2 [Staphylococcus aureus subsp. aureus COL],3DWK_D Chain D, Penicillin-binding protein 2 [Staphylococcus aureus subsp. aureus COL] |
| 2JE5_A | 1.06e-29 | 36 | 599 | 29 | 675 | StructuralAnd Mechanistic Basis Of Penicillin Binding Protein Inhibition By Lactivicins [Streptococcus pneumoniae R6],2JE5_B Structural And Mechanistic Basis Of Penicillin Binding Protein Inhibition By Lactivicins [Streptococcus pneumoniae R6] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P76577 | 2.13e-75 | 65 | 749 | 68 | 767 | Penicillin-binding protein 1C OS=Escherichia coli (strain K12) OX=83333 GN=pbpC PE=1 SV=1 |
| P38050 | 8.78e-48 | 2 | 581 | 3 | 623 | Penicillin-binding protein 1F OS=Bacillus subtilis (strain 168) OX=224308 GN=pbpF PE=2 SV=2 |
| Q89AR2 | 2.06e-40 | 30 | 553 | 104 | 678 | Penicillin-binding protein 1B OS=Buchnera aphidicola subsp. Baizongia pistaciae (strain Bp) OX=224915 GN=mrcB PE=3 SV=1 |
| O66874 | 3.34e-40 | 43 | 537 | 47 | 639 | Penicillin-binding protein 1A OS=Aquifex aeolicus (strain VF5) OX=224324 GN=mrcA PE=1 SV=1 |
| P40750 | 4.46e-40 | 45 | 540 | 60 | 599 | Penicillin-binding protein 4 OS=Bacillus subtilis (strain 168) OX=224308 GN=pbpD PE=1 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
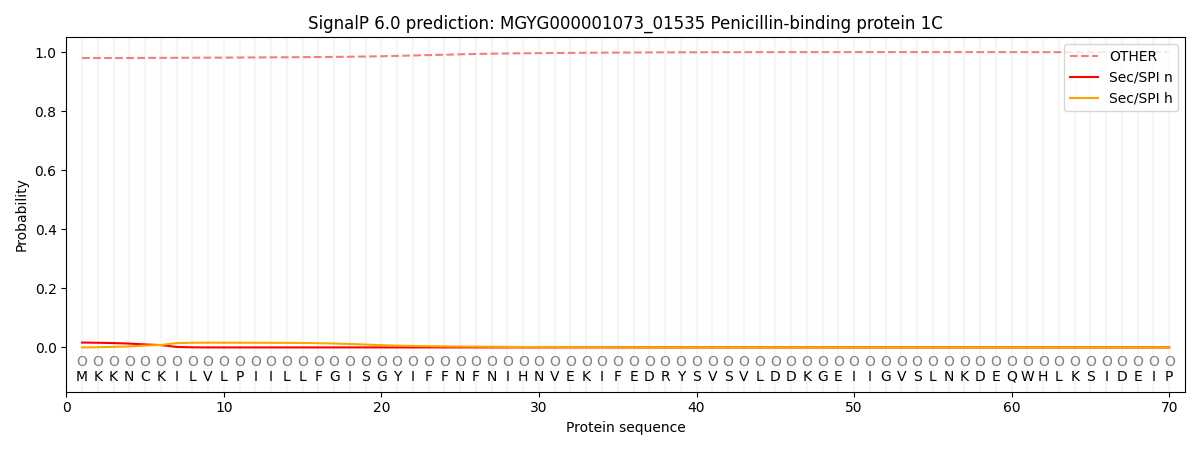
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.980859 | 0.015221 | 0.002756 | 0.000093 | 0.000053 | 0.001045 |