You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001160_00538
You are here: Home > Sequence: MGYG000001160_00538
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | CAG-274 sp000432155 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Lachnospirales; CAG-274; CAG-274; CAG-274 sp000432155 | |||||||||||
| CAZyme ID | MGYG000001160_00538 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 37918; End: 42837 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL1 | 248 | 451 | 8.6e-33 | 0.8663366336633663 |
| CBM77 | 744 | 832 | 6.3e-18 | 0.8737864077669902 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3866 | PelB | 1.74e-27 | 188 | 533 | 46 | 343 | Pectate lyase [Carbohydrate transport and metabolism]. |
| smart00656 | Amb_all | 1.14e-19 | 257 | 454 | 12 | 190 | Amb_all domain. |
| cd14256 | Dockerin_I | 2.17e-14 | 1047 | 1101 | 1 | 55 | Type I dockerin repeat domain. Bacterial cohesin domains bind to a complementary protein domain named dockerin, and this interaction is required for the formation of the cellulosome, a cellulose-degrading complex. The cellulosome consists of scaffoldin, a noncatalytic scaffolding polypeptide, that comprises repeating cohesion modules and a single carbohydrate-binding module (CBM). Specific calcium-dependent interactions between cohesins and dockerins appear to be essential for cellulosome assembly. This subfamily represents type I dockerins, which are responsible for anchoring a variety of enzymatic domains to the complex. |
| pfam18283 | CBM77 | 2.36e-13 | 745 | 837 | 13 | 108 | Carbohydrate binding module 77. This domain is the non-catalytic carbohydrate binding module 77 (CBM77) present in Ruminococcus flavefaciens. CBMs fulfil a critical targeting function in plant cell wall depolymerisation. In CBM77, a cluster of conserved basic residues (Lys1092, Lys1107 and Lys1162) confer calcium-independent recognition of homogalacturonan. |
| pfam00404 | Dockerin_1 | 3.48e-12 | 1048 | 1101 | 1 | 54 | Dockerin type I repeat. The dockerin repeat is the binding partner of the cohesin domain pfam00963. The cohesin-dockerin interaction is the crucial interaction for complex formation in the cellulosome. The dockerin repeats, each bearing homology to the EF-hand calcium-binding loop bind calcium. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BBF42492.1 | 2.98e-152 | 6 | 698 | 5 | 673 |
| ACR71161.1 | 2.64e-124 | 34 | 843 | 41 | 895 |
| ADU23076.1 | 1.26e-113 | 38 | 510 | 761 | 1209 |
| CDR31241.1 | 1.86e-85 | 38 | 669 | 333 | 893 |
| QQY82326.1 | 3.62e-64 | 21 | 840 | 17 | 750 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3VMV_A | 1.31e-15 | 256 | 450 | 74 | 246 | Crystalstructure of pectate lyase Bsp165PelA from Bacillus sp. N165 [Bacillus sp. N16-5],3VMW_A Crystal structure of pectate lyase Bsp165PelA from Bacillus sp. N165 in complex with trigalacturonate [Bacillus sp. N16-5] |
| 2QX3_A | 2.60e-14 | 255 | 537 | 66 | 330 | Structureof pectate lyase II from Xanthomonas campestris pv. campestris str. ATCC 33913 [Xanthomonas campestris pv. campestris],2QX3_B Structure of pectate lyase II from Xanthomonas campestris pv. campestris str. ATCC 33913 [Xanthomonas campestris pv. campestris] |
| 2QY1_A | 2.01e-13 | 255 | 537 | 66 | 330 | ChainA, Pectate lyase II [Xanthomonas campestris pv. campestris],2QY1_B Chain B, Pectate lyase II [Xanthomonas campestris pv. campestris] |
| 2QXZ_A | 2.01e-13 | 255 | 537 | 66 | 330 | ChainA, pectate lyase II [Xanthomonas campestris pv. campestris],2QXZ_B Chain B, pectate lyase II [Xanthomonas campestris pv. campestris] |
| 5AMV_A | 3.66e-13 | 261 | 458 | 127 | 326 | Structuralinsights into the loss of catalytic competence in pectate lyase at low pH [Bacillus subtilis],5X2I_A Polygalacturonate Lyase by Fusing with a Self-assembling Amphipathic Peptide [Bacillus subtilis subsp. subtilis str. 168] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8GCB2 | 2.79e-14 | 255 | 459 | 102 | 281 | Pectate trisaccharide-lyase OS=Bacillus licheniformis OX=1402 GN=pelA PE=1 SV=1 |
| Q65DC2 | 2.79e-14 | 255 | 459 | 102 | 281 | Pectate trisaccharide-lyase OS=Bacillus licheniformis (strain ATCC 14580 / DSM 13 / JCM 2505 / CCUG 7422 / NBRC 12200 / NCIMB 9375 / NCTC 10341 / NRRL NRS-1264 / Gibson 46) OX=279010 GN=BLi04129 PE=3 SV=1 |
| B1B6T1 | 2.79e-14 | 255 | 459 | 102 | 281 | Pectate trisaccharide-lyase OS=Bacillus sp. OX=1409 GN=pel PE=1 SV=1 |
| P39116 | 2.29e-12 | 261 | 458 | 148 | 347 | Pectate lyase OS=Bacillus subtilis (strain 168) OX=224308 GN=pel PE=1 SV=1 |
| Q6CZT2 | 1.65e-11 | 337 | 533 | 163 | 363 | Pectate lyase 3 OS=Pectobacterium atrosepticum (strain SCRI 1043 / ATCC BAA-672) OX=218491 GN=pel3 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
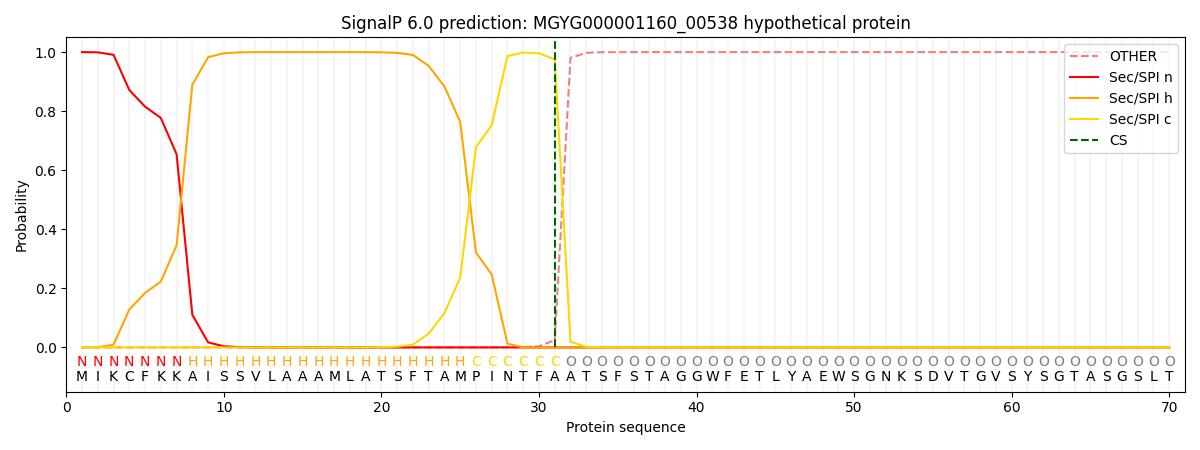
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000292 | 0.998957 | 0.000175 | 0.000223 | 0.000177 | 0.000153 |

