You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001265_00366
You are here: Home > Sequence: MGYG000001265_00366
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
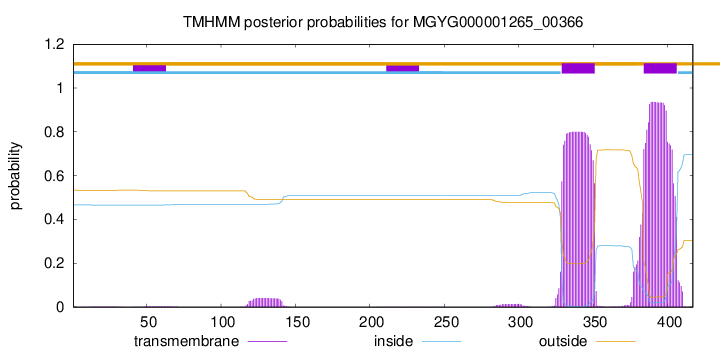
TMHMM annotations
Basic Information help
| Species | Methylobacterium sp002778835 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Proteobacteria; Alphaproteobacteria; Rhizobiales; Beijerinckiaceae; Methylobacterium; Methylobacterium sp002778835 | |||||||||||
| CAZyme ID | MGYG000001265_00366 | |||||||||||
| CAZy Family | GH104 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 27142; End: 28395 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH104 | 12 | 149 | 6.8e-18 | 0.8620689655172413 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd00736 | lambda_lys-like | 2.99e-12 | 20 | 150 | 16 | 127 | Bacteriophage lambda lysozyme and similar proteins. Lysozyme from bacteriophage lambda hydrolyzes the beta-1,4-glycosidic bond between N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc), as do other lysozymes. However, unlike other lysozymes, bacteriophage lambda does not produce a reducing end upon cleavage of the peptidoglycan, but rather uses the 6-OH of the same MurNAc residue to produce a 1,6-anhydromuramic acid terminal residue and is therefore a lytic transglycosylase. An identical 1,6-anhydro bond is formed in bacterial peptidoglycans by the action of the lytic transglycosylases of E. coli, though they differ structurally. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QFI66838.1 | 2.61e-69 | 6 | 199 | 1 | 200 |
| QND60148.1 | 7.68e-66 | 5 | 179 | 4 | 172 |
| AZO04594.1 | 1.38e-65 | 6 | 342 | 5 | 302 |
| ASY63459.1 | 2.49e-65 | 6 | 210 | 5 | 214 |
| QFP61877.1 | 4.76e-62 | 6 | 208 | 5 | 199 |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as OTHER
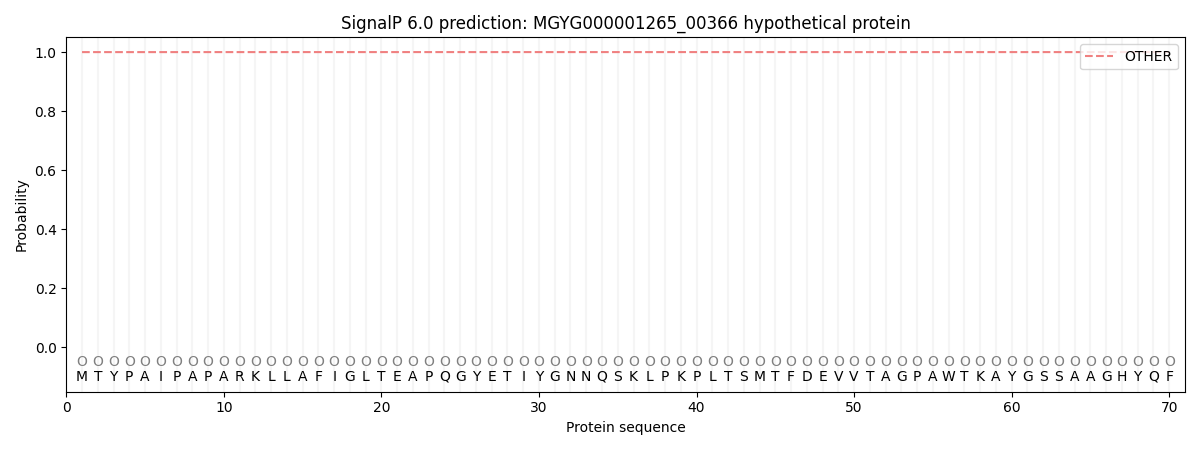
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 1.000011 | 0.000020 | 0.000000 | 0.000000 | 0.000000 | 0.000000 |