You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001345_01896
You are here: Home > Sequence: MGYG000001345_01896
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
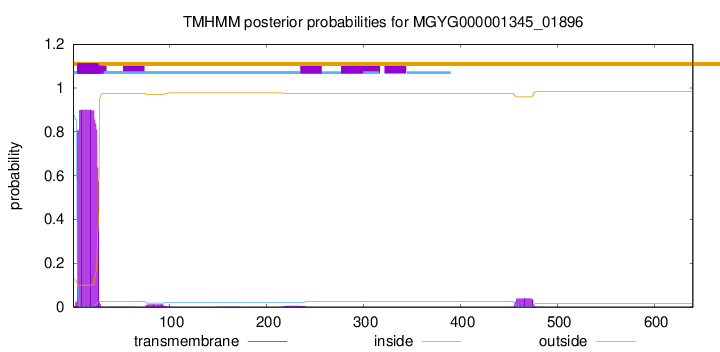
TMHMM annotations
Basic Information help
| Species | Bacteroides xylanisolvens | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides xylanisolvens | |||||||||||
| CAZyme ID | MGYG000001345_01896 | |||||||||||
| CAZy Family | GH28 | |||||||||||
| CAZyme Description | 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 286660; End: 288582 Strand: + | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG0596 | MhpC | 1.11e-35 | 374 | 639 | 5 | 282 | Pimeloyl-ACP methyl ester carboxylesterase [Coenzyme transport and metabolism, General function prediction only]. |
| pfam00144 | Beta-lactamase | 2.42e-33 | 35 | 327 | 1 | 300 | Beta-lactamase. This family appears to be distantly related to pfam00905 and PF00768 D-alanyl-D-alanine carboxypeptidase. |
| TIGR02427 | protocat_pcaD | 1.32e-29 | 381 | 637 | 2 | 251 | 3-oxoadipate enol-lactonase. Members of this family are 3-oxoadipate enol-lactonase. Note that the substrate is known as 3-oxoadipate enol-lactone, 2-oxo-2,3-dihydrofuran-5-acetate, 4,5-Dihydro-5-oxofuran-2-acetate, and 5-oxo-4,5-dihydrofuran-2-acetate. The enzyme the catalyzes the fourth step in the protocatechuate degradation to beta-ketoadipate and then to succinyl-CoA and acetyl-CoA. 4-hydroxybenzoate, 3-hydroxybenzoate, and vanillate all can be converted in one step to protocatechuate. This enzyme also acts in catechol degradation. In genomes that catabolize both catechol and protocatechuate, two forms of this enzyme may be found. All members of the seed alignment for this model were chosen from within protocatechuate degradation operons of at least three genes of the pathway, from genomes with the complete pathway through beta-ketoadipate. [Energy metabolism, Other] |
| COG1680 | AmpC | 4.89e-27 | 4 | 328 | 8 | 356 | CubicO group peptidase, beta-lactamase class C family [Defense mechanisms]. |
| PRK14875 | PRK14875 | 2.00e-20 | 373 | 639 | 113 | 371 | acetoin dehydrogenase E2 subunit dihydrolipoyllysine-residue acetyltransferase; Provisional |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUT92203.1 | 9.80e-150 | 362 | 637 | 511 | 786 |
| ALJ62047.1 | 1.95e-149 | 362 | 637 | 511 | 786 |
| BBM69122.1 | 1.27e-17 | 35 | 257 | 606 | 864 |
| BBM72115.1 | 1.27e-17 | 35 | 257 | 606 | 864 |
| QXD14887.1 | 1.68e-17 | 35 | 257 | 600 | 859 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5TGF_A | 5.43e-183 | 24 | 362 | 2 | 337 | ChainA, Uncharacterized protein [Phocaeicola dorei DSM 17855],5TGF_B Chain B, Uncharacterized protein [Phocaeicola dorei DSM 17855],5TGF_C Chain C, Uncharacterized protein [Phocaeicola dorei DSM 17855],5TGF_D Chain D, Uncharacterized protein [Phocaeicola dorei DSM 17855] |
| 5EGN_A | 2.39e-19 | 374 | 636 | 4 | 260 | Est816as an N-Acyl homoserine lactone degrading enzyme [uncultured bacterium],5EGN_B Est816 as an N-Acyl homoserine lactone degrading enzyme [uncultured bacterium],5EGN_C Est816 as an N-Acyl homoserine lactone degrading enzyme [uncultured bacterium],5EGN_D Est816 as an N-Acyl homoserine lactone degrading enzyme [uncultured bacterium],5EGN_E Est816 as an N-Acyl homoserine lactone degrading enzyme [uncultured bacterium],5EGN_F Est816 as an N-Acyl homoserine lactone degrading enzyme [uncultured bacterium],5EGN_G Est816 as an N-Acyl homoserine lactone degrading enzyme [uncultured bacterium],5EGN_H Est816 as an N-Acyl homoserine lactone degrading enzyme [uncultured bacterium] |
| 3FOB_A | 7.02e-19 | 381 | 638 | 19 | 281 | Crystalstructure of bromoperoxidase from Bacillus anthracis [Bacillus anthracis str. Ames],3FOB_B Crystal structure of bromoperoxidase from Bacillus anthracis [Bacillus anthracis str. Ames],3FOB_C Crystal structure of bromoperoxidase from Bacillus anthracis [Bacillus anthracis str. Ames] |
| 1HKH_A | 2.04e-16 | 372 | 486 | 1 | 124 | unligatedgamma lactamase from an Aureobacterium species [Microbacterium],1HKH_B unligated gamma lactamase from an Aureobacterium species [Microbacterium],1HL7_A Gamma lactamase from an Aureobacterium species in complex with 3a,4,7,7a-tetrahydro-benzo [1,3] dioxol-2-one [Microbacterium sp.],1HL7_B Gamma lactamase from an Aureobacterium species in complex with 3a,4,7,7a-tetrahydro-benzo [1,3] dioxol-2-one [Microbacterium sp.] |
| 4IQ4_A | 2.61e-16 | 381 | 640 | 16 | 280 | Structureof a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P21212 form [Kitasatospora aureofaciens],4IQ4_B Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P21212 form [Kitasatospora aureofaciens],4IQ4_C Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P21212 form [Kitasatospora aureofaciens],4IQ4_D Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P21212 form [Kitasatospora aureofaciens],4IQ4_E Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P21212 form [Kitasatospora aureofaciens],4IQ4_F Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P21212 form [Kitasatospora aureofaciens],4ITV_A Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P212121 form [Kitasatospora aureofaciens],4ITV_B Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P212121 form [Kitasatospora aureofaciens],4ITV_C Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P212121 form [Kitasatospora aureofaciens],4ITV_D Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P212121 form [Kitasatospora aureofaciens],4ITV_E Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P212121 form [Kitasatospora aureofaciens],4ITV_F Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P212121 form [Kitasatospora aureofaciens],4ITV_G Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P212121 form [Kitasatospora aureofaciens],4ITV_H Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P212121 form [Kitasatospora aureofaciens],4ITV_I Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P212121 form [Kitasatospora aureofaciens],4ITV_J Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P212121 form [Kitasatospora aureofaciens],4ITV_K Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P212121 form [Kitasatospora aureofaciens],4ITV_L Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, P212121 form [Kitasatospora aureofaciens],4IVJ_A Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, I222 form [Kitasatospora aureofaciens],4IVJ_B Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, I222 form [Kitasatospora aureofaciens],4IVJ_C Structure of a 16 nm protein cage designed by fusing symmetric oligomeric domains, triple mutant, I222 form [Kitasatospora aureofaciens] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q55921 | 7.16e-17 | 381 | 637 | 16 | 276 | Putative non-heme chloroperoxidase OS=Synechocystis sp. (strain PCC 6803 / Kazusa) OX=1111708 GN=slr0314 PE=3 SV=1 |
| P29715 | 1.49e-15 | 381 | 636 | 16 | 276 | Non-haem bromoperoxidase BPO-A2 OS=Kitasatospora aureofaciens OX=1894 GN=bpoA2 PE=1 SV=3 |
| O31168 | 6.63e-15 | 381 | 636 | 16 | 276 | Non-heme chloroperoxidase OS=Kitasatospora aureofaciens OX=1894 GN=cpo PE=1 SV=1 |
| P33912 | 6.93e-14 | 381 | 636 | 12 | 273 | Non-heme chloroperoxidase CPO-A1 OS=Kitasatospora aureofaciens OX=1894 GN=bpoA1 PE=1 SV=3 |
| Q59093 | 1.49e-13 | 392 | 640 | 30 | 266 | 3-oxoadipate enol-lactonase 1 OS=Acinetobacter baylyi (strain ATCC 33305 / BD413 / ADP1) OX=62977 GN=pcaD PE=4 SV=2 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
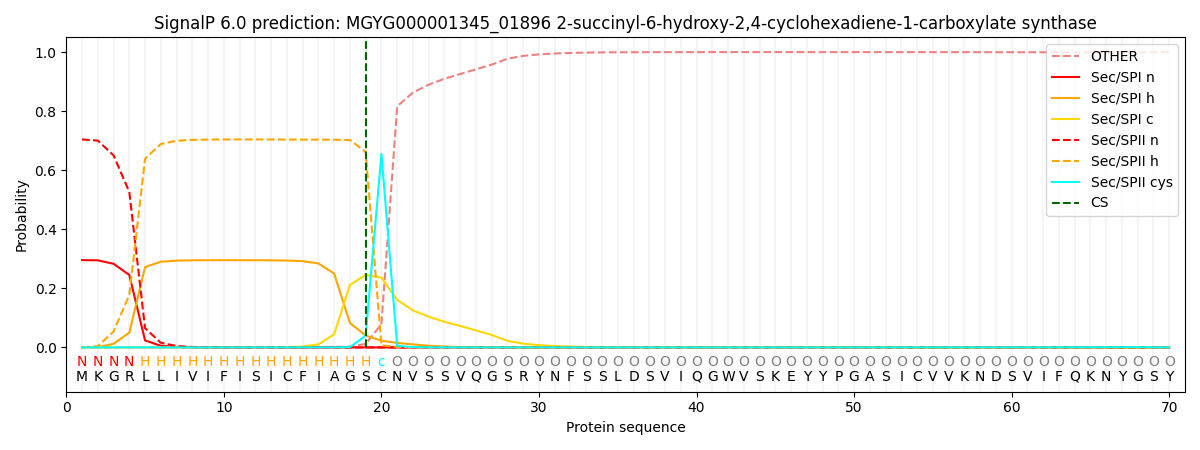
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000334 | 0.290653 | 0.708670 | 0.000119 | 0.000117 | 0.000108 |