You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001370_01658
You are here: Home > Sequence: MGYG000001370_01658
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides fluxus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides fluxus | |||||||||||
| CAZyme ID | MGYG000001370_01658 | |||||||||||
| CAZy Family | CE3 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 48716; End: 49984 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE3 | 197 | 417 | 5.8e-18 | 0.9845360824742269 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam03629 | SASA | 3.41e-40 | 23 | 192 | 3 | 226 | Carbohydrate esterase, sialic acid-specific acetylesterase. The catalytic triad of this esterase enzyme comprises residues Ser127, His403 and Asp391 in UniProtKB:P70665. |
| cd01834 | SGNH_hydrolase_like_2 | 1.53e-39 | 196 | 417 | 1 | 190 | SGNH_hydrolase subfamily. SGNH hydrolases are a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the Ser-His-Asp(Glu) triad found in other serine hydrolases. |
| pfam13472 | Lipase_GDSL_2 | 3.11e-27 | 201 | 410 | 1 | 176 | GDSL-like Lipase/Acylhydrolase family. This family of presumed lipases and related enzymes are similar to pfam00657. |
| cd00229 | SGNH_hydrolase | 9.86e-21 | 199 | 417 | 1 | 186 | SGNH_hydrolase, or GDSL_hydrolase, is a diverse family of lipases and esterases. The tertiary fold of the enzyme is substantially different from that of the alpha/beta hydrolase family and unique among all known hydrolases; its active site closely resembles the typical Ser-His-Asp(Glu) triad from other serine hydrolases, but may lack the carboxlic acid. |
| COG2755 | TesA | 2.15e-16 | 198 | 422 | 10 | 211 | Lysophospholipase L1 or related esterase [Amino acid transport and metabolism]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUT78436.1 | 4.26e-46 | 21 | 192 | 30 | 260 |
| QDM11351.1 | 4.26e-46 | 21 | 192 | 30 | 260 |
| QRM98824.1 | 4.26e-46 | 21 | 192 | 30 | 260 |
| QUT32559.1 | 5.97e-46 | 5 | 192 | 10 | 260 |
| QDH54197.1 | 8.38e-46 | 22 | 192 | 31 | 260 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4Q9A_A | 1.45e-29 | 199 | 421 | 22 | 228 | Crystalstructure of a putative GDSL-like lipase (PARMER_00689) from Parabacteroides merdae ATCC 43184 at 2.86 A resolution [Parabacteroides merdae ATCC 43184],4Q9A_B Crystal structure of a putative GDSL-like lipase (PARMER_00689) from Parabacteroides merdae ATCC 43184 at 2.86 A resolution [Parabacteroides merdae ATCC 43184] |
| 3W7V_A | 7.89e-28 | 195 | 420 | 5 | 212 | CrystalStructure of Axe2, an Acetylxylan Esterase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],3W7V_B Crystal Structure of Axe2, an Acetylxylan Esterase from Geobacillus stearothermophilus [Geobacillus stearothermophilus] |
| 5BN1_A | 7.89e-28 | 195 | 420 | 5 | 212 | Structureof Axe2-W215I, an acetyl xylan esterase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],5BN1_B Structure of Axe2-W215I, an acetyl xylan esterase from Geobacillus stearothermophilus [Geobacillus stearothermophilus] |
| 4OAO_A | 7.89e-28 | 195 | 420 | 5 | 212 | Amutant of Axe2 (R55A), and acetyl-xylooligosaccharide esterase from Geobacillus Stearmophilus [Geobacillus stearothermophilus],4OAO_B A mutant of Axe2 (R55A), and acetyl-xylooligosaccharide esterase from Geobacillus Stearmophilus [Geobacillus stearothermophilus] |
| 4JKO_A | 2.09e-27 | 195 | 420 | 5 | 212 | Crystalstructure of a catalytic mutant of Axe2 (Axe2_S15A), an acetylxylan esterase from Geobacillus stearothermophilus [Geobacillus stearothermophilus],4JKO_B Crystal structure of a catalytic mutant of Axe2 (Axe2_S15A), an acetylxylan esterase from Geobacillus stearothermophilus [Geobacillus stearothermophilus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q09LX1 | 4.32e-27 | 195 | 420 | 5 | 212 | Acetylxylan esterase OS=Geobacillus stearothermophilus OX=1422 GN=axe2 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
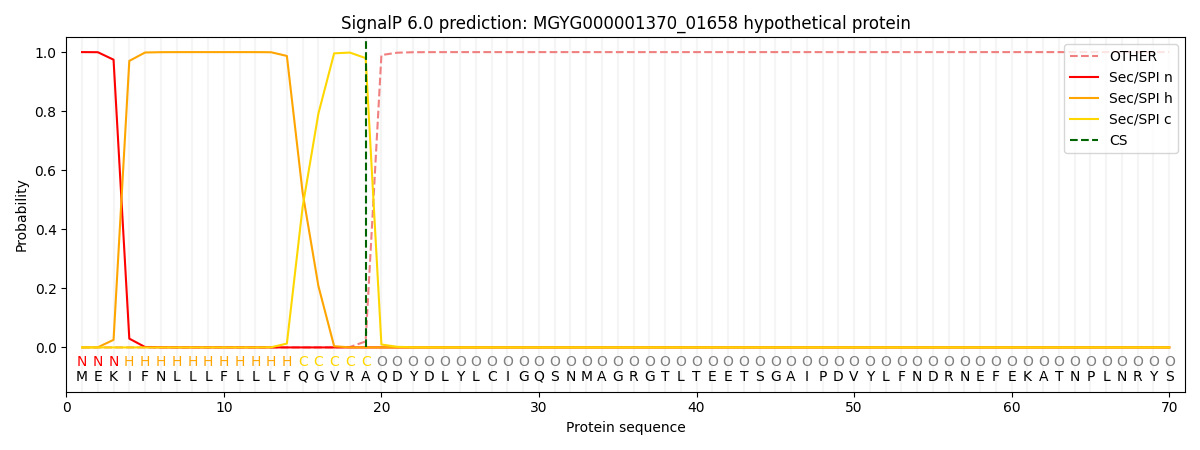
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000212 | 0.999198 | 0.000155 | 0.000142 | 0.000135 | 0.000133 |
