You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001375_01010
You are here: Home > Sequence: MGYG000001375_01010
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Ruminococcus_F champanellensis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Oscillospirales; Ruminococcaceae; Ruminococcus_F; Ruminococcus_F champanellensis | |||||||||||
| CAZyme ID | MGYG000001375_01010 | |||||||||||
| CAZy Family | GH146 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 1141618; End: 1144845 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH146 | 45 | 568 | 8.2e-176 | 0.9940357852882704 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam07944 | Glyco_hydro_127 | 2.18e-135 | 43 | 568 | 1 | 503 | Beta-L-arabinofuranosidase, GH127. One member of this family, from Bidobacterium longicum, UniProtKB:E8MGH8, has been characterized as an unusual beta-L-arabinofuranosidase enzyme, EC:3.2.1.185. It rleases l-arabinose from the l-arabinofuranose (Araf)-beta1,2-Araf disaccharide and also transglycosylates 1-alkanols with retention of the anomeric configuration. Terminal beta-l-arabinofuranosyl residues have been found in arabinogalactan proteins from a mumber of different plantt species. beta-l-Arabinofuranosyl linkages with 1-4 arabinofuranosides are also found in the sugar chains of extensin and solanaceous lectins, hydroxyproline (Hyp)2-rich glycoproteins that are widely observed in plant cell wall fractions. The critical residue for catalytic activity is Glu-338, in a ET/SCAS sequence context. |
| COG3533 | COG3533 | 3.76e-95 | 44 | 572 | 12 | 505 | Uncharacterized conserved protein, DUF1680 family [Function unknown]. |
| cd14256 | Dockerin_I | 2.18e-16 | 1020 | 1072 | 1 | 54 | Type I dockerin repeat domain. Bacterial cohesin domains bind to a complementary protein domain named dockerin, and this interaction is required for the formation of the cellulosome, a cellulose-degrading complex. The cellulosome consists of scaffoldin, a noncatalytic scaffolding polypeptide, that comprises repeating cohesion modules and a single carbohydrate-binding module (CBM). Specific calcium-dependent interactions between cohesins and dockerins appear to be essential for cellulosome assembly. This subfamily represents type I dockerins, which are responsible for anchoring a variety of enzymatic domains to the complex. |
| pfam00404 | Dockerin_1 | 3.01e-14 | 1021 | 1072 | 1 | 53 | Dockerin type I repeat. The dockerin repeat is the binding partner of the cohesin domain pfam00963. The cohesin-dockerin interaction is the crucial interaction for complex formation in the cellulosome. The dockerin repeats, each bearing homology to the EF-hand calcium-binding loop bind calcium. |
| cd14253 | Dockerin | 7.66e-09 | 1021 | 1072 | 1 | 53 | Dockerin repeat domain. Dockerins are modules in the cellulosome complex that often anchor catalytic subunits by binding to cohesin domains of scaffolding proteins. Three types of dockerins and their corresponding cohesin have been described in the literature. This alignment models two consecutive dockerin repeats, the functional unit. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CBL17203.1 | 0.0 | 1 | 1075 | 1 | 1075 |
| ADL33431.1 | 2.18e-266 | 36 | 815 | 2 | 777 |
| AZK46778.1 | 2.85e-215 | 36 | 778 | 12 | 732 |
| QSF44176.1 | 2.40e-209 | 36 | 780 | 16 | 738 |
| AIQ16786.1 | 5.17e-207 | 36 | 784 | 16 | 746 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6YQH_AAA | 2.09e-94 | 39 | 792 | 28 | 792 | ChainAAA, Acetyl-CoA carboxylase, biotin carboxylase [Bacteroides thetaiotaomicron VPI-5482] |
| 5OPJ_A | 1.82e-91 | 39 | 792 | 28 | 792 | Beta-L-arabinofuranosidase[Bacteroides thetaiotaomicron] |
| 5MQO_A | 6.98e-16 | 187 | 609 | 196 | 644 | Glycosidehydrolase BT_1003 [Bacteroides thetaiotaomicron] |
| 3WRE_A | 2.49e-07 | 184 | 514 | 145 | 508 | Thecrystal structure of native HypBA1 from Bifidobacterium longum JCM 1217 [Bifidobacterium longum subsp. longum JCM 1217],3WRG_A The complex structure of HypBA1 with L-arabinose [Bifidobacterium longum subsp. longum JCM 1217] |
| 3WKW_A | 2.51e-07 | 184 | 514 | 145 | 508 | Crystalstructure of GH127 beta-L-arabinofuranosidase HypBA1 from Bifidobacterium longum ligand free form [Bifidobacterium longum subsp. longum JCM 1217],3WKX_A Crystal structure of GH127 beta-L-arabinofuranosidase HypBA1 from Bifidobacterium longum arabinose complex form [Bifidobacterium longum subsp. longum JCM 1217],7BZL_A Chain A, Non-reducing end beta-L-arabinofuranosidase [Bifidobacterium longum subsp. longum JCM 1217],7DIF_A Chain A, Non-reducing end beta-L-arabinofuranosidase [Bifidobacterium longum subsp. longum JCM 1217],7EXV_A Chain A, Non-reducing end beta-L-arabinofuranosidase [Bifidobacterium longum subsp. longum JCM 1217],7EXW_A Chain A, Non-reducing end beta-L-arabinofuranosidase [Bifidobacterium longum subsp. longum JCM 1217] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| E8MGH8 | 1.37e-06 | 184 | 514 | 145 | 508 | Non-reducing end beta-L-arabinofuranosidase OS=Bifidobacterium longum subsp. longum (strain ATCC 15707 / DSM 20219 / JCM 1217 / NCTC 11818 / E194b) OX=565042 GN=hypBA1 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
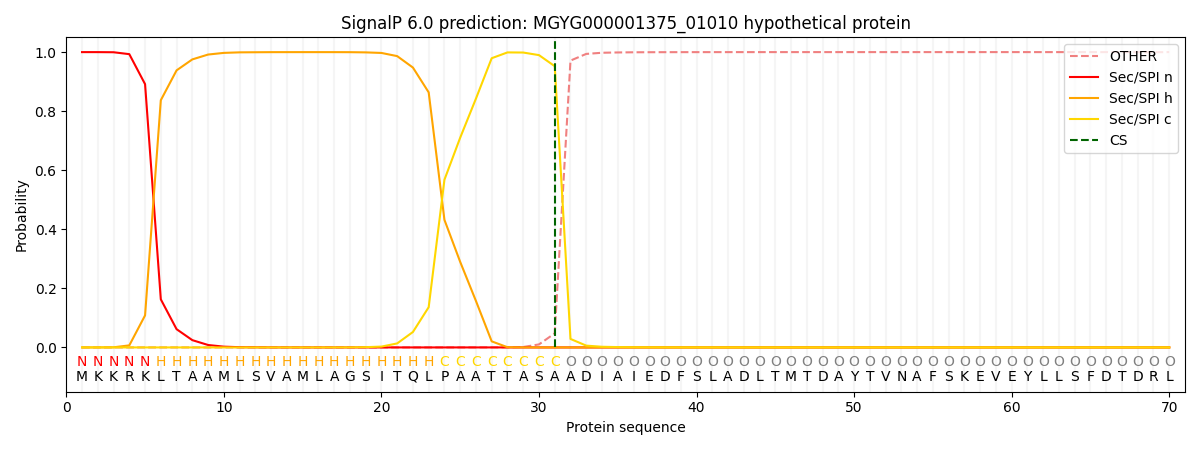
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000452 | 0.998488 | 0.000275 | 0.000317 | 0.000221 | 0.000190 |
