You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001378_00132
You are here: Home > Sequence: MGYG000001378_00132
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
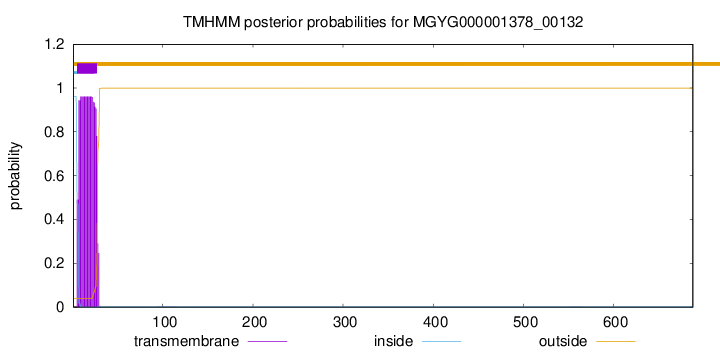
TMHMM annotations
Basic Information help
| Species | Bacteroides ovatus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides ovatus | |||||||||||
| CAZyme ID | MGYG000001378_00132 | |||||||||||
| CAZy Family | GH36 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 178792; End: 180858 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH36 | 167 | 416 | 4.8e-28 | 0.3633720930232558 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd14791 | GH36 | 2.37e-22 | 312 | 525 | 4 | 244 | glycosyl hydrolase family 36 (GH36). GH36 enzymes occur in prokaryotes, eukaryotes, and archaea with a wide range of hydrolytic activities, including alpha-galactosidase, alpha-N-acetylgalactosaminidase, stachyose synthase, and raffinose synthase. All GH36 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH36 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| pfam02065 | Melibiase | 1.10e-11 | 279 | 412 | 7 | 130 | Melibiase. Glycoside hydrolase families GH27, GH31 and GH36 form the glycoside hydrolase clan GH-D. Glycoside hydrolase family 36 can be split into 11 families, GH36A to GH36K. This family includes enzymes from GH36A-B and GH36D-K and from GH27. |
| COG3345 | GalA | 1.71e-08 | 312 | 416 | 294 | 385 | Alpha-galactosidase [Carbohydrate transport and metabolism]. |
| cd14790 | GH_D | 5.68e-06 | 316 | 469 | 7 | 148 | Glycoside hydrolases, clan D. This group of glycosyl hydrolase families is comprised of glycosyl hydrolase family 31 (GH31), family 36 (GH36), and family 27 (GH27). These structurally and mechanistically related protein families are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. They have a wide range of functions including alpha-glucosidase, alpha-xylosidase, 6-alpha-glucosyltransferase, 3-alpha-isomaltosyltransferase, alpha-N-acetylgalactosaminidase, stachyose synthase, raffinose synthase, and alpha-1,4-glucan lyase. |
| cd14792 | GH27 | 2.36e-05 | 315 | 400 | 6 | 84 | glycosyl hydrolase family 27 (GH27). GH27 enzymes occur in eukaryotes, prokaryotes, and archaea with a wide range of hydrolytic activities, including alpha-glucosidase (glucoamylase and sucrase-isomaltase), alpha-N-acetylgalactosaminidase, and 3-alpha-isomalto-dextranase. All GH27 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH27 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QJS14159.1 | 5.72e-13 | 290 | 419 | 277 | 393 |
| ACU71470.1 | 5.79e-13 | 293 | 421 | 281 | 411 |
| ALV53775.1 | 5.88e-13 | 301 | 419 | 301 | 404 |
| QCD60939.1 | 7.54e-13 | 290 | 419 | 277 | 393 |
| AJG06892.1 | 1.02e-12 | 301 | 419 | 301 | 404 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6JHP_A | 3.83e-11 | 156 | 421 | 205 | 475 | Crystalstructure of the glycoside hydrolase family 36 alpha-galactosidase from Paecilomyces thermophila [Paecilomyces sp. 'thermophila'],6JHP_B Crystal structure of the glycoside hydrolase family 36 alpha-galactosidase from Paecilomyces thermophila [Paecilomyces sp. 'thermophila'],6JHP_C Crystal structure of the glycoside hydrolase family 36 alpha-galactosidase from Paecilomyces thermophila [Paecilomyces sp. 'thermophila'],6JHP_D Crystal structure of the glycoside hydrolase family 36 alpha-galactosidase from Paecilomyces thermophila [Paecilomyces sp. 'thermophila'] |
| 3MI6_A | 2.51e-07 | 285 | 420 | 304 | 430 | ChainA, Alpha-galactosidase [Levilactobacillus brevis ATCC 367],3MI6_B Chain B, Alpha-galactosidase [Levilactobacillus brevis ATCC 367],3MI6_C Chain C, Alpha-galactosidase [Levilactobacillus brevis ATCC 367],3MI6_D Chain D, Alpha-galactosidase [Levilactobacillus brevis ATCC 367] |
| 2XN0_A | 5.67e-07 | 296 | 420 | 319 | 430 | Structureof alpha-galactosidase from Lactobacillus acidophilus NCFM, PtCl4 derivative [Lactobacillus acidophilus NCFM],2XN0_B Structure of alpha-galactosidase from Lactobacillus acidophilus NCFM, PtCl4 derivative [Lactobacillus acidophilus NCFM],2XN1_A Structure of alpha-galactosidase from Lactobacillus acidophilus NCFM with TRIS [Lactobacillus acidophilus NCFM],2XN1_B Structure of alpha-galactosidase from Lactobacillus acidophilus NCFM with TRIS [Lactobacillus acidophilus NCFM],2XN1_C Structure of alpha-galactosidase from Lactobacillus acidophilus NCFM with TRIS [Lactobacillus acidophilus NCFM],2XN1_D Structure of alpha-galactosidase from Lactobacillus acidophilus NCFM with TRIS [Lactobacillus acidophilus NCFM] |
| 2XN2_A | 5.67e-07 | 296 | 420 | 319 | 430 | Structureof alpha-galactosidase from Lactobacillus acidophilus NCFM with galactose [Lactobacillus acidophilus NCFM] |
| 4FNQ_A | 2.23e-06 | 312 | 410 | 331 | 416 | Crystalstructure of GH36 alpha-galactosidase AgaB from Geobacillus stearothermophilus [Geobacillus stearothermophilus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q0CVH2 | 3.03e-11 | 278 | 421 | 313 | 463 | Probable alpha-galactosidase C OS=Aspergillus terreus (strain NIH 2624 / FGSC A1156) OX=341663 GN=aglC PE=3 SV=1 |
| Q5AU92 | 1.20e-10 | 295 | 421 | 335 | 467 | Alpha-galactosidase C OS=Emericella nidulans (strain FGSC A4 / ATCC 38163 / CBS 112.46 / NRRL 194 / M139) OX=227321 GN=aglC PE=1 SV=1 |
| B8NWY6 | 1.89e-09 | 295 | 421 | 335 | 466 | Probable alpha-galactosidase C OS=Aspergillus flavus (strain ATCC 200026 / FGSC A1120 / IAM 13836 / NRRL 3357 / JCM 12722 / SRRC 167) OX=332952 GN=aglC PE=3 SV=2 |
| Q2TW69 | 1.89e-09 | 295 | 421 | 335 | 466 | Probable alpha-galactosidase C OS=Aspergillus oryzae (strain ATCC 42149 / RIB 40) OX=510516 GN=aglC PE=3 SV=1 |
| Q9UUZ4 | 6.71e-08 | 295 | 412 | 332 | 448 | Alpha-galactosidase C OS=Aspergillus niger OX=5061 GN=aglC PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
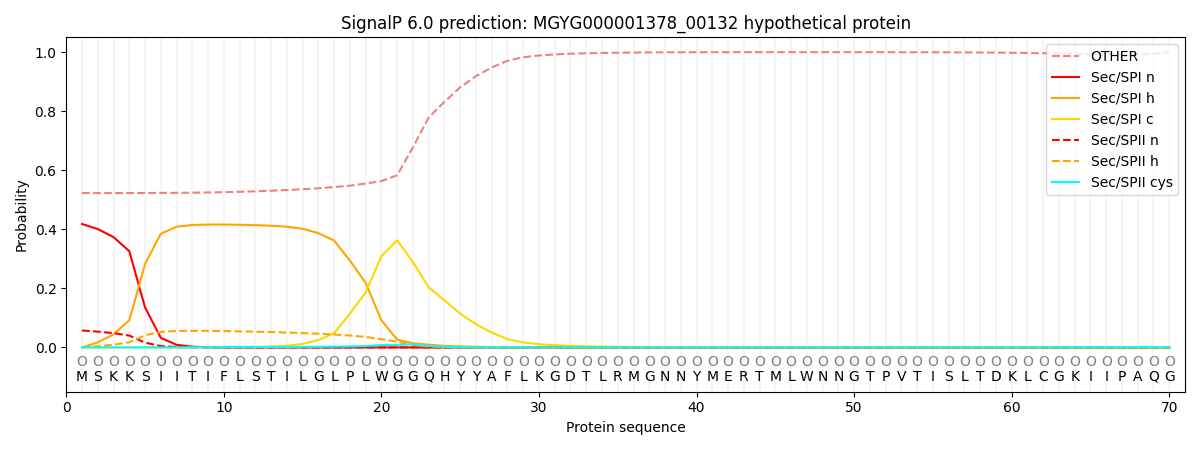
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.540432 | 0.392658 | 0.060832 | 0.001632 | 0.000928 | 0.003521 |