You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001393_01880
You are here: Home > Sequence: MGYG000001393_01880
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Hafnia paralvei | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Proteobacteria; Gammaproteobacteria; Enterobacterales; Enterobacteriaceae; Hafnia; Hafnia paralvei | |||||||||||
| CAZyme ID | MGYG000001393_01880 | |||||||||||
| CAZy Family | GH24 | |||||||||||
| CAZyme Description | Lysozyme RrrD | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 8456; End: 8983 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH24 | 26 | 164 | 2.3e-31 | 0.948905109489051 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3772 | RrrD | 1.06e-43 | 15 | 174 | 1 | 152 | Phage-related lysozyme (muramidase), GH24 family [Cell wall/membrane/envelope biogenesis]. |
| cd16900 | endolysin_R21-like | 1.46e-43 | 17 | 170 | 1 | 142 | endolysin R21-like proteins. Unlike T4 E phage lysozyme, the endolysin R21 from Enterobacteria phage P21 has an N-terminal SAR (signal-arrest-release) domain that anchors the endolysin to the membrane in an inactive form, which act to prevent premature lysis of the infected bacterium. The dsDNA phages of eubacteria use endolysins or muralytic enzymes in conjunction with hollin, a small membrane protein, to degrade the peptidoglycan found in bacterial cell walls. Similarly, bacteria produce autolysins to facilitate the biosynthesis of its cell wall heteropolymer peptidoglycan and cell division. Endolysins and autolysins are found in viruses and bacteria, respectively. Both endolysin and autolysin enzymes cleave the glycosidic beta 1,4-bonds between the N-acetylmuramic acid and the N-acetylglucosamine of the peptidoglycan. |
| cd00737 | lyz_endolysin_autolysin | 1.12e-23 | 27 | 146 | 3 | 119 | endolysin and autolysin. The dsDNA phages of eubacteria use endolysins or muralytic enzymes in conjunction with hollin, a small membrane protein, to degrade the peptidoglycan found in bacterial cell walls. Similarly, bacteria produce autolysins to facilitate the biosynthesis of its cell wall heteropolymer peptidoglycan and cell division. Endolysins and autolysins are found in viruses and bacteria, respectively. Both endolysin and autolysin enzymes cleave the glycosidic beta 1,4-bonds between the N-acetylmuramic acid and the N-acetylglucosamine of the peptidoglycan. |
| cd16901 | lyz_P1 | 6.48e-16 | 33 | 168 | 14 | 138 | P1 lysozyme Lyz-like proteins. Enterobacteria phage P1 lysozyme Lyz is secreted to the Escherichia coli periplasm where it is membrane bound and inactive. Activation involves the release from the membrane, an intramolecular thiol-disulfide isomerization and extensive structural rearrangement of the N-terminal region. The dsDNA phages of eubacteria use endolysins or muralytic enzymes in conjunction with hollin, a small membrane protein, to degrade the peptidoglycan found in bacterial cell walls. Similarly, bacteria produce autolysins to facilitate the biosynthesis of its cell wall heteropolymer peptidoglycan and cell division. Endolysins and autolysins are found in viruses and bacteria, respectively. Both endolysin and autolysin enzymes cleave the glycosidic beta 1,4-bonds between the N-acetylmuramic acid and the N-acetylglucosamine of the peptidoglycan. |
| pfam00959 | Phage_lysozyme | 2.10e-15 | 46 | 162 | 1 | 106 | Phage lysozyme. This family includes lambda phage lysozyme and E. coli endolysin. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QQE41734.1 | 2.33e-130 | 1 | 175 | 1 | 175 |
| QQE41783.1 | 3.06e-126 | 1 | 175 | 1 | 175 |
| QIP56296.1 | 4.18e-124 | 1 | 175 | 1 | 175 |
| AMH20069.1 | 2.50e-123 | 1 | 175 | 1 | 176 |
| QDW32871.1 | 2.20e-92 | 4 | 175 | 5 | 176 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3HDE_A | 1.38e-17 | 7 | 172 | 5 | 163 | ChainA, Lysozyme [Enterobacteria phage P21],3HDE_B Chain B, Lysozyme [Enterobacteria phage P21],3HDE_C Chain C, Lysozyme [Enterobacteria phage P21],3HDE_D Chain D, Lysozyme [Enterobacteria phage P21] |
| 4ZPU_A | 1.72e-17 | 7 | 172 | 5 | 163 | Thestructure of DLP12 endolysin exhibits likely active and inactive conformations. [Escherichia coli K-12],4ZPU_B The structure of DLP12 endolysin exhibits likely active and inactive conformations. [Escherichia coli K-12],4ZPU_C The structure of DLP12 endolysin exhibits likely active and inactive conformations. [Escherichia coli K-12],4ZPU_D The structure of DLP12 endolysin exhibits likely active and inactive conformations. [Escherichia coli K-12] |
| 3HDF_A | 2.16e-17 | 30 | 172 | 7 | 138 | ChainA, Lysozyme [Enterobacteria phage P21],3HDF_B Chain B, Lysozyme [Enterobacteria phage P21] |
| 7M5I_A | 3.43e-17 | 33 | 140 | 21 | 126 | ChainA, Endolysin [Escherichia coli O157 typing phage 15],7M5I_B Chain B, Endolysin [Escherichia coli O157 typing phage 15] |
| 6ET6_A | 1.58e-12 | 7 | 146 | 38 | 177 | ChainA, Lysozyme [Acinetobacter baumannii] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P68920 | 1.69e-88 | 7 | 175 | 9 | 177 | SAR-endolysin OS=Escherichia phage 933W OX=10730 GN=R PE=3 SV=1 |
| P68921 | 1.69e-88 | 7 | 175 | 9 | 177 | SAR-endolysin OS=Enterobacteria phage VT2-Sa OX=97081 GN=R PE=3 SV=1 |
| P76159 | 1.96e-87 | 1 | 175 | 1 | 177 | Probable prophage lysozyme OS=Escherichia coli (strain K12) OX=83333 GN=rrrQ PE=3 SV=1 |
| O64362 | 1.94e-85 | 1 | 175 | 1 | 178 | SAR-endolysin OS=Escherichia phage N15 OX=40631 GN=54 PE=3 SV=1 |
| Q9ZXB7 | 3.78e-85 | 7 | 175 | 9 | 177 | SAR-endolysin OS=Enterobacteria phage H19B OX=69932 GN=R PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
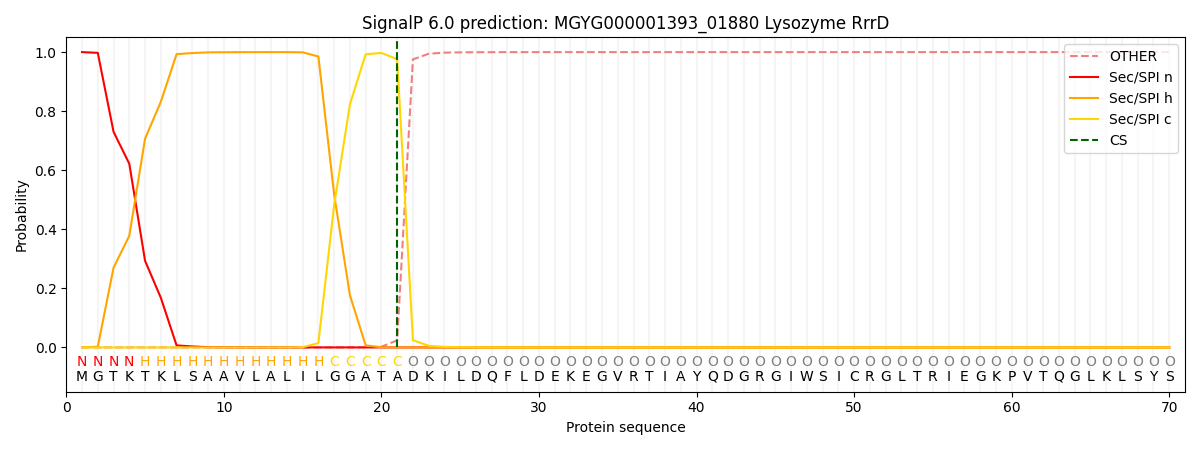
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000316 | 0.999088 | 0.000151 | 0.000146 | 0.000134 | 0.000131 |
