You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001422_00752
You are here: Home > Sequence: MGYG000001422_00752
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Bacteroides oleiciplenus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides oleiciplenus | |||||||||||
| CAZyme ID | MGYG000001422_00752 | |||||||||||
| CAZy Family | GH18 | |||||||||||
| CAZyme Description | Endo-beta-N-acetylglucosaminidase | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 840796; End: 841743 Strand: + | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd06542 | GH18_EndoS-like | 1.60e-36 | 49 | 284 | 1 | 240 | Endo-beta-N-acetylglucosaminidases are bacterial chitinases that hydrolyze the chitin core of various asparagine (N)-linked glycans and glycoproteins. The endo-beta-N-acetylglucosaminidases have a glycosyl hydrolase family 18 (GH18) catalytic domain. Some members also have an additional C-terminal glycosyl hydrolase family 20 (GH20) domain while others have an N-terminal domain of unknown function (pfam08522). Members of this family include endo-beta-N-acetylglucosaminidase S (EndoS) from Streptococcus pyogenes, EndoF1, EndoF2, EndoF3, and EndoH from Flavobacterium meningosepticum, and EndoE from Enterococcus faecalis. EndoS is a secreted endoglycosidase from Streptococcus pyogenes that specifically hydrolyzes the glycan on human IgG between two core N-acetylglucosamine residues. EndoE is a secreted endoglycosidase, encoded by the ndoE gene in Enterococcus faecalis, that hydrolyzes the glycan on human RNase B. |
| pfam00704 | Glyco_hydro_18 | 3.31e-10 | 83 | 221 | 27 | 158 | Glycosyl hydrolases family 18. |
| cd00598 | GH18_chitinase-like | 4.20e-07 | 53 | 225 | 3 | 164 | The GH18 (glycosyl hydrolase, family 18) type II chitinases hydrolyze chitin, an abundant polymer of beta-1,4-linked N-acetylglucosamine (GlcNAc) which is a major component of the cell wall of fungi and the exoskeleton of arthropods. Chitinases have been identified in viruses, bacteria, fungi, protozoan parasites, insects, and plants. The structure of the GH18 domain is an eight-stranded beta/alpha barrel with a pronounced active-site cleft at the C-terminal end of the beta-barrel. The GH18 family includes chitotriosidase, chitobiase, hevamine, zymocin-alpha, narbonin, SI-CLP (stabilin-1 interacting chitinase-like protein), IDGF (imaginal disc growth factor), CFLE (cortical fragment-lytic enzyme) spore hydrolase, the type III and type V plant chitinases, the endo-beta-N-acetylglucosaminidases, and the chitolectins. The GH85 (glycosyl hydrolase, family 85) ENGases (endo-beta-N-acetylglucosaminidases) are closely related to the GH18 chitinases and are included in this alignment model. |
| cd02878 | GH18_zymocin_alpha | 2.26e-06 | 143 | 208 | 88 | 156 | Zymocin, alpha subunit. Zymocin is a heterotrimeric enzyme that inhibits yeast cell cycle progression. The zymocin alpha subunit has a chitinase activity that is essential for holoenzyme action from the cell exterior while the gamma subunit contains the intracellular toxin responsible for G1 phase cell cycle arrest. The zymocin alpha and beta subunits are thought to act from the cell's exterior by docking to the cell wall-associated chitin, thus mediating gamma-toxin translocation. The alpha subunit has an eight-stranded TIM barrel fold similar to that of family 18 glycosyl hydrolases such as hevamine, chitolectin, and chitobiase. |
| cd06548 | GH18_chitinase | 5.45e-04 | 142 | 228 | 105 | 201 | The GH18 (glycosyl hydrolases, family 18) type II chitinases hydrolyze chitin, an abundant polymer of N-acetylglucosamine and have been identified in bacteria, fungi, insects, plants, viruses, and protozoan parasites. The structure of this domain is an eight-stranded alpha/beta barrel with a pronounced active-site cleft at the C-terminal end of the beta-barrel. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QDM12014.1 | 3.60e-176 | 1 | 315 | 1 | 315 |
| BCA50677.1 | 1.81e-122 | 40 | 315 | 14 | 286 |
| BCA50675.1 | 7.30e-105 | 34 | 315 | 9 | 286 |
| QUT39058.1 | 1.00e-64 | 1 | 315 | 1 | 306 |
| QQA10383.1 | 1.00e-64 | 1 | 315 | 1 | 306 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 1EDT_A | 5.92e-36 | 49 | 304 | 9 | 268 | ChainA, ENDO-BETA-N-ACETYLGLUCOSAMINIDASE H, ENDO H [Streptomyces plicatus] |
| 6VE1_A | 7.51e-36 | 49 | 304 | 13 | 272 | ChainA, Endo-beta-N-acetylglucosaminidase H [Streptomyces plicatus],6VE1_B Chain B, Endo-beta-N-acetylglucosaminidase H [Streptomyces plicatus],6VE1_C Chain C, Endo-beta-N-acetylglucosaminidase H [Streptomyces plicatus],6VE1_D Chain D, Endo-beta-N-acetylglucosaminidase H [Streptomyces plicatus] |
| 1C90_A | 1.41e-35 | 49 | 304 | 4 | 263 | ChainA, ENDO-BETA-N-ACETYLGLUCOSAMINIDASE H [Streptomyces plicatus],1C90_B Chain B, ENDO-BETA-N-ACETYLGLUCOSAMINIDASE H [Streptomyces plicatus] |
| 1C91_A | 1.41e-35 | 49 | 304 | 4 | 263 | ChainA, ENDO-BETA-N-ACETYLGLUCOSAMINIDASE H [Streptomyces plicatus] |
| 1C8X_A | 1.97e-35 | 49 | 304 | 4 | 263 | ChainA, ENDO-BETA-N-ACETYLGLUCOSAMINIDASE H [Streptomyces plicatus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P80036 | 1.31e-39 | 49 | 306 | 55 | 313 | Endo-beta-N-acetylglucosaminidase OS=Flavobacterium sp. (strain SK1022) OX=148444 PE=1 SV=2 |
| P04067 | 8.48e-35 | 49 | 304 | 51 | 310 | Endo-beta-N-acetylglucosaminidase H OS=Streptomyces plicatus OX=1922 PE=1 SV=1 |
| P36911 | 1.59e-30 | 50 | 228 | 61 | 242 | Endo-beta-N-acetylglucosaminidase F1 OS=Elizabethkingia meningoseptica OX=238 GN=endOF1 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
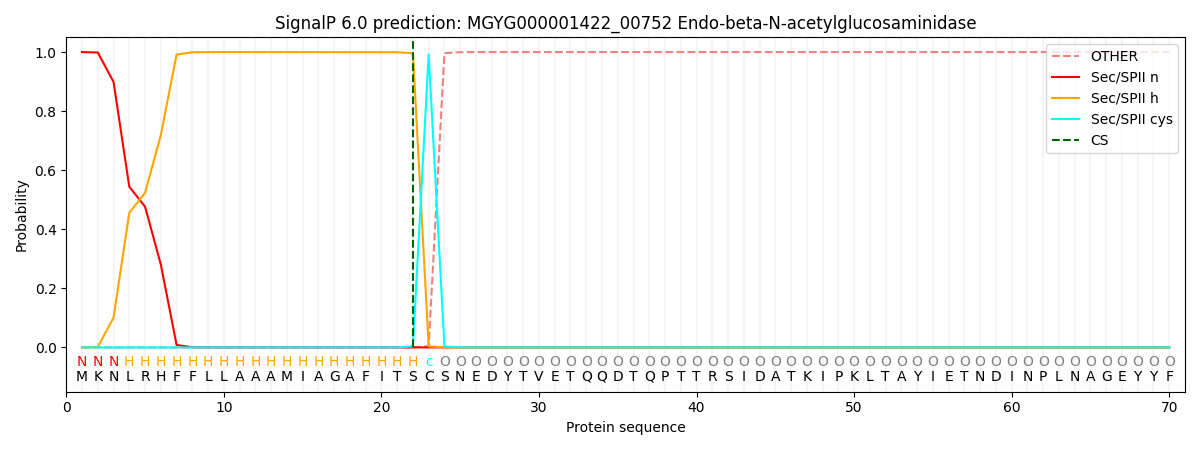
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000000 | 0.000000 | 1.000065 | 0.000000 | 0.000000 | 0.000000 |
