You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001422_02617
You are here: Home > Sequence: MGYG000001422_02617
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
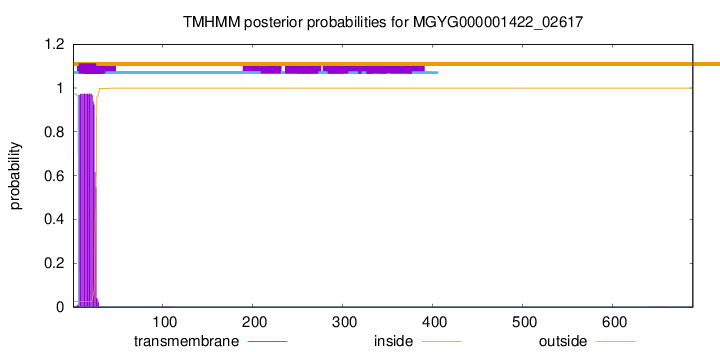
TMHMM annotations
Basic Information help
| Species | Bacteroides oleiciplenus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Bacteroidaceae; Bacteroides; Bacteroides oleiciplenus | |||||||||||
| CAZyme ID | MGYG000001422_02617 | |||||||||||
| CAZy Family | GH42 | |||||||||||
| CAZyme Description | Beta-galactosidase BgaA | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 1301820; End: 1303889 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH42 | 37 | 401 | 5.9e-119 | 0.9919137466307277 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam02449 | Glyco_hydro_42 | 1.17e-91 | 37 | 403 | 2 | 376 | Beta-galactosidase. This group of beta-galactosidase enzymes belong to the glycosyl hydrolase 42 family. The enzyme catalyzes the hydrolysis of terminal, non-reducing terminal beta-D-galactosidase residues. |
| COG1874 | GanA | 2.66e-77 | 31 | 689 | 16 | 672 | Beta-galactosidase GanA [Carbohydrate transport and metabolism]. |
| pfam08532 | Glyco_hydro_42M | 2.59e-35 | 414 | 618 | 1 | 206 | Beta-galactosidase trimerisation domain. This is non catalytic domain B of beta-galactosidase enzymes belong to the glycosyl hydrolase 42 family. This domain is related to glutamine amidotransferase enzymes, but the catalytic residues are replaced by non functional amino acids. This domain is involved in trimerisation. |
| cd03143 | A4_beta-galactosidase_middle_domain | 1.03e-17 | 416 | 618 | 1 | 154 | A4 beta-galactosidase middle domain: a type 1 glutamine amidotransferase (GATase1)-like domain. A4 beta-galactosidase middle domain: a type 1 glutamine amidotransferase (GATase1)-like domain. This group includes proteins similar to beta-galactosidase from Thermus thermophilus. Beta-Galactosidase hydrolyzes the beta-1,4-D-galactosidic linkage of lactose, as well as those of related chromogens, o-nitrophenyl-beta-D-galactopyranoside (ONP-Gal) and 5-bromo-4-chloro-3-indolyl-beta-D-galactoside (X-gal). This A4 beta-galactosidase middle domain lacks the catalytic triad of typical GATase1 domains. The reactive Cys residue found in the sharp turn between a beta strand and an alpha helix termed the nucleophile elbow in typical GATase1 domains is not conserved in this group. |
| cd10938 | CE4_HpPgdA_like | 0.006 | 68 | 125 | 29 | 76 | Catalytic domain of Helicobacter pylori peptidoglycan deacetylase (HpPgdA) and similar proteins. This family is represented by a peptidoglycan deacetylase (HP0310, HpPgdA) from the gram-negative pathogen Helicobacter pylori. HpPgdA has the ability to bind a metal ion at the active site and is responsible for a peptidoglycan modification that counteracts the host immune response. It functions as a homotetramer. The monomer is composed of a 7-stranded barrel with detectable sequence similarity to the 6-stranded barrel NodB homology domain of polysaccharide deacetylase (DCA)-like proteins in the CE4 superfamily, which removes N-linked or O-linked acetyl groups from cell wall polysaccharides. In contrast to typical NodB-like DCAs, HpPgdA does not exhibit a solvent-accessible polysaccharide binding groove, suggesting that the enzyme binds a small molecule at the active site. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| ALJ60205.1 | 0.0 | 1 | 689 | 1 | 689 |
| QUT88793.1 | 0.0 | 1 | 689 | 1 | 689 |
| QQA30410.1 | 0.0 | 10 | 688 | 7 | 684 |
| QUT61938.1 | 0.0 | 10 | 688 | 7 | 684 |
| BBK86027.1 | 0.0 | 10 | 688 | 7 | 684 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5XB7_A | 3.72e-138 | 29 | 687 | 7 | 693 | GH42alpha-L-arabinopyranosidase from Bifidobacterium animalis subsp. lactis Bl-04 [Bifidobacterium animalis subsp. lactis],5XB7_B GH42 alpha-L-arabinopyranosidase from Bifidobacterium animalis subsp. lactis Bl-04 [Bifidobacterium animalis subsp. lactis],5XB7_C GH42 alpha-L-arabinopyranosidase from Bifidobacterium animalis subsp. lactis Bl-04 [Bifidobacterium animalis subsp. lactis],5XB7_D GH42 alpha-L-arabinopyranosidase from Bifidobacterium animalis subsp. lactis Bl-04 [Bifidobacterium animalis subsp. lactis],5XB7_E GH42 alpha-L-arabinopyranosidase from Bifidobacterium animalis subsp. lactis Bl-04 [Bifidobacterium animalis subsp. lactis],5XB7_F GH42 alpha-L-arabinopyranosidase from Bifidobacterium animalis subsp. lactis Bl-04 [Bifidobacterium animalis subsp. lactis] |
| 3TTS_A | 2.02e-66 | 27 | 672 | 5 | 659 | ChainA, Beta-galactosidase [Niallia circulans subsp. alkalophilus],3TTS_B Chain B, Beta-galactosidase [Niallia circulans subsp. alkalophilus],3TTS_C Chain C, Beta-galactosidase [Niallia circulans subsp. alkalophilus],3TTS_D Chain D, Beta-galactosidase [Niallia circulans subsp. alkalophilus],3TTS_E Chain E, Beta-galactosidase [Niallia circulans subsp. alkalophilus],3TTS_F Chain F, Beta-galactosidase [Niallia circulans subsp. alkalophilus],3TTY_A Chain A, Beta-galactosidase [Niallia circulans subsp. alkalophilus],3TTY_B Chain B, Beta-galactosidase [Niallia circulans subsp. alkalophilus],3TTY_C Chain C, Beta-galactosidase [Niallia circulans subsp. alkalophilus],3TTY_D Chain D, Beta-galactosidase [Niallia circulans subsp. alkalophilus],3TTY_E Chain E, Beta-galactosidase [Niallia circulans subsp. alkalophilus],3TTY_F Chain F, Beta-galactosidase [Niallia circulans subsp. alkalophilus] |
| 5E9A_A | 9.15e-64 | 18 | 655 | 29 | 678 | Crystalstructure analysis of the cold-adamped beta-galactosidase from Rahnella sp. R3 [Rahnella sp. R3],5E9A_B Crystal structure analysis of the cold-adamped beta-galactosidase from Rahnella sp. R3 [Rahnella sp. R3],5E9A_C Crystal structure analysis of the cold-adamped beta-galactosidase from Rahnella sp. R3 [Rahnella sp. R3],5E9A_D Crystal structure analysis of the cold-adamped beta-galactosidase from Rahnella sp. R3 [Rahnella sp. R3],5E9A_E Crystal structure analysis of the cold-adamped beta-galactosidase from Rahnella sp. R3 [Rahnella sp. R3],5E9A_F Crystal structure analysis of the cold-adamped beta-galactosidase from Rahnella sp. R3 [Rahnella sp. R3] |
| 6LVW_A | 4.60e-61 | 34 | 618 | 4 | 613 | PolyextremophilicBeta-galactosidase from the Antarctic haloarchaeon Halorubrum lacusprofundi [Halorubrum lacusprofundi ATCC 49239] |
| 1KWG_A | 2.30e-59 | 34 | 689 | 3 | 644 | Crystalstructure of Thermus thermophilus A4 beta-galactosidase [Thermus thermophilus],1KWK_A Crystal structure of Thermus thermophilus A4 beta-galactosidase in complex with galactose [Thermus thermophilus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| D9SM34 | 1.19e-79 | 34 | 688 | 4 | 656 | Beta-galactosidase BgaA OS=Clostridium cellulovorans (strain ATCC 35296 / DSM 3052 / OCM 3 / 743B) OX=573061 GN=bgaA PE=1 SV=1 |
| P19668 | 2.28e-72 | 29 | 689 | 5 | 665 | Beta-galactosidase bgaB OS=Geobacillus kaustophilus OX=1462 GN=bgaB PE=1 SV=1 |
| C9S0R2 | 3.15e-71 | 29 | 689 | 5 | 665 | Beta-galactosidase BgaB OS=Geobacillus sp. (strain Y412MC61) OX=544556 GN=bgaB PE=3 SV=1 |
| C8WV58 | 2.84e-67 | 19 | 659 | 6 | 656 | Beta-galactosidase BglY OS=Alicyclobacillus acidocaldarius subsp. acidocaldarius (strain ATCC 27009 / DSM 446 / BCRC 14685 / JCM 5260 / KCTC 1825 / NBRC 15652 / NCIMB 11725 / NRRL B-14509 / 104-IA) OX=521098 GN=bglY PE=1 SV=1 |
| Q0TUR6 | 1.06e-66 | 27 | 654 | 10 | 656 | Beta-galactosidase Pbg OS=Clostridium perfringens (strain ATCC 13124 / DSM 756 / JCM 1290 / NCIMB 6125 / NCTC 8237 / Type A) OX=195103 GN=pbg PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
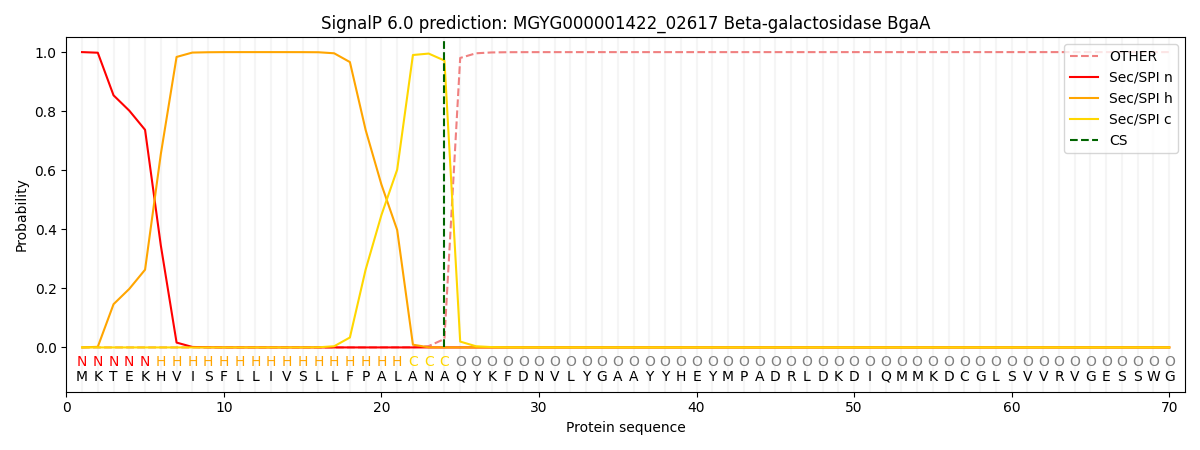
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000226 | 0.999096 | 0.000196 | 0.000165 | 0.000155 | 0.000147 |