You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001489_00694
You are here: Home > Sequence: MGYG000001489_00694
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Parabacteroides goldsteinii | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Tannerellaceae; Parabacteroides; Parabacteroides goldsteinii | |||||||||||
| CAZyme ID | MGYG000001489_00694 | |||||||||||
| CAZy Family | GH97 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 822846; End: 825881 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH97 | 17 | 624 | 9.2e-138 | 0.9841521394611727 |
| GH117 | 836 | 987 | 2.2e-16 | 0.7298578199052133 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd08994 | GH43_62_32_68_117_130-like | 1.56e-91 | 655 | 987 | 4 | 294 | Glycosyl hydrolase families: GH43, GH62, GH32, GH68, GH117, CH130. Members of the glycosyl hydrolase families 32, 43, 62, 68, 117 and 130 (GH32, GH43, GH62, GH68, GH117, GH130) all possess 5-bladed beta-propeller domains and comprise clans F and J, as classified by the carbohydrate-active enzymes database (CAZY). Clan F consists of families GH43 and GH62. GH43 includes beta-xylosidases (EC 3.2.1.37), beta-xylanases (EC 3.2.1.8), alpha-L-arabinases (EC 3.2.1.99), and alpha-L-arabinofuranosidases (EC 3.2.1.55), using aryl-glycosides as substrates, while family GH62 contains alpha-L-arabinofuranosidases (EC 3.2.1.55) that specifically cleave either alpha-1,2 or alpha-1,3-L-arabinofuranose sidechains from xylans. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Clan J consists of families GH32 and GH68. GH32 comprises sucrose-6-phosphate hydrolases, invertases (EC 3.2.1.26), inulinases (EC 3.2.1.7), levanases (EC 3.2.1.65), eukaryotic fructosyltransferases, and bacterial fructanotransferases while GH68 consists of frucosyltransferases (FTFs) that include levansucrase (EC 2.4.1.10); beta-fructofuranosidase (EC 3.2.1.26); inulosucrase (EC 2.4.1.9), while GH68 consists of frucosyltransferases (FTFs) that include levansucrase (EC 2.4.1.10); beta-fructofuranosidase (EC 3.2.1.26); inulosucrase (EC 2.4.1.9), all of which use sucrose as their preferential donor substrate. Members of this clan are retaining enzymes (i.e. they retain the configuration at anomeric carbon atom of the substrate) that catalyze hydrolysis in two steps involving a covalent glycosyl enzyme intermediate: an aspartate located close to the N-terminus acts as the catalytic nucleophile and a glutamate acts as the general acid/base; a conserved aspartate residue in the Arg-Asp-Pro (RDP) motif stabilizes the transition state. Structures of all families in the two clans manifest a funnel-shaped active site that comprises two subsites with a single route for access by ligands. Also included in this superfamily are GH117 enzymes that have exo-alpha-1,3-(3,6-anhydro)-l-galactosidase activity, removing terminal non-reducing alpha-1,3-linked 3,6-anhydro-l-galactose residues from their neoagarose substrate, and GH130 that are phosphorylases and hydrolases for beta-mannosides, involved in the bacterial utilization of mannans or N-linked glycans. |
| pfam10566 | Glyco_hydro_97 | 1.39e-51 | 269 | 490 | 2 | 255 | Glycoside hydrolase 97. This domain is the catalytic region of the bacterial glycosyl-hydrolase family 97. This central part of the GH97 family protein sequences represents a typical and complete (beta/alpha)8-barrel or catalytic TIM-barrel type domain. The N- and C-terminal parts of the sequences, mainly consisting of beta-strands, form two additional non-catalytic domains. In all known glycosidases with the (beta-alpha)8-barrel fold, the amino acid residues at the active site are located on the C-termini of the beta-strands. |
| pfam14508 | GH97_N | 4.33e-51 | 27 | 263 | 1 | 235 | Glycosyl-hydrolase 97 N-terminal. This N-terminal domain of glycosyl-hydrolase-97 contributes part of the active site pocket. It is also important for contact with the catalytic and C-terminal domains of the whole. |
| pfam14509 | GH97_C | 1.41e-19 | 531 | 625 | 1 | 97 | Glycosyl-hydrolase 97 C-terminal, oligomerization. Glycosyl-hydrolase-97 is made up of three tightly linked and highly conserved globular domains. The C-terminal domain is found to be necessary for oligomerization of the whole molecule in order to create the active-site pocket and the Ca++-binding site. |
| cd08992 | GH117 | 2.38e-11 | 673 | 879 | 30 | 216 | Glycosyl hydrolase family 117 (GH117). This glycoside hydrolase 117 (GH117) family includes alpha-1,3-L-neoagarooligosaccharide hydrolase (EC 3.2.1.-); alpha-1,3-L-neoagarobiase/neoagarobiose hydrolase (NABH, EC 3.2.1.-). In the agarolytic pathway, in order to metabolize agar, NABH is an essential enzyme because it converts alpha-neoagarobiose (O-3,6-anhydro-alpha-l-galactopyranosyl-(1,3)-d-galactose) into fermentable monosaccharides (d-galactose and 3,6-anhydro-l-galactose). Thus, these enzymes have exo-alpha-1,3-(3,6-anhydro)-l-galactosidase activity, removing terminal non-reducing alpha-1,3-linked 3,6-anhydro-l-galactose residues from their neoagarose substrate. This family includes Zobellia galactanivorans enzymes, Zg4663 and Zg3615 (also known as ZgAhgA and ZgAhgB, respectively) that have been shown to have similar activity on unsubstituted agarose oligosaccharides while Zg3597 has been shown to be inactive, possibly due to differences in dimerization conformation, active-site structure and function. GH117 shares distant sequence similarity with families GH43 and GH32. A common structural feature of all these enzymes is a 5-bladed beta-propeller domain, similar to GH43, that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QJR68579.1 | 1.22e-238 | 622 | 1010 | 21 | 409 |
| QJR72910.1 | 1.22e-238 | 622 | 1010 | 21 | 409 |
| QJR64313.1 | 1.22e-238 | 622 | 1010 | 21 | 409 |
| QUT84599.1 | 2.78e-238 | 622 | 1010 | 15 | 403 |
| AND21092.1 | 2.06e-234 | 622 | 1010 | 21 | 409 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5E1Q_A | 9.53e-55 | 27 | 586 | 20 | 614 | Mutant(D415G) GH97 alpha-galactosidase in complex with Gal-Lac [Bacteroides thetaiotaomicron VPI-5482],5E1Q_B Mutant (D415G) GH97 alpha-galactosidase in complex with Gal-Lac [Bacteroides thetaiotaomicron VPI-5482] |
| 3A24_A | 6.31e-54 | 27 | 586 | 6 | 600 | Crystalstructure of BT1871 retaining glycosidase [Bacteroides thetaiotaomicron],3A24_B Crystal structure of BT1871 retaining glycosidase [Bacteroides thetaiotaomicron] |
| 3WFA_A | 5.68e-45 | 66 | 626 | 69 | 705 | Catalyticrole of the calcium ion in GH97 inverting glycoside hydrolase [Bacteroides thetaiotaomicron],3WFA_B Catalytic role of the calcium ion in GH97 inverting glycoside hydrolase [Bacteroides thetaiotaomicron] |
| 2JKA_A | 5.73e-45 | 66 | 626 | 78 | 714 | Nativestructure of a family 97 alpha-glucosidase from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482],2JKA_B Native structure of a family 97 alpha-glucosidase from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482],2JKE_A Structure of a family 97 alpha-glucosidase from Bacteroides thetaiotaomicron in complex with deoxynojirimycin [Bacteroides thetaiotaomicron VPI-5482],2JKE_B Structure of a family 97 alpha-glucosidase from Bacteroides thetaiotaomicron in complex with deoxynojirimycin [Bacteroides thetaiotaomicron VPI-5482],2JKP_A Structure of a family 97 alpha-glucosidase from Bacteroides thetaiotaomicron in complex with castanospermine [Bacteroides thetaiotaomicron VPI-5482],2JKP_B Structure of a family 97 alpha-glucosidase from Bacteroides thetaiotaomicron in complex with castanospermine [Bacteroides thetaiotaomicron VPI-5482] |
| 2D73_A | 6.35e-45 | 66 | 626 | 89 | 725 | CrystalStructure Analysis of SusB [Bacteroides thetaiotaomicron VPI-5482],2D73_B Crystal Structure Analysis of SusB [Bacteroides thetaiotaomicron VPI-5482],2ZQ0_A Crystal structure of SusB complexed with acarbose [Bacteroides thetaiotaomicron],2ZQ0_B Crystal structure of SusB complexed with acarbose [Bacteroides thetaiotaomicron] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8A6L0 | 9.51e-55 | 1 | 586 | 1 | 621 | Retaining alpha-galactosidase OS=Bacteroides thetaiotaomicron (strain ATCC 29148 / DSM 2079 / JCM 5827 / CCUG 10774 / NCTC 10582 / VPI-5482 / E50) OX=226186 GN=BT_1871 PE=1 SV=1 |
| G8JZS4 | 3.48e-44 | 66 | 626 | 89 | 725 | Glucan 1,4-alpha-glucosidase SusB OS=Bacteroides thetaiotaomicron (strain ATCC 29148 / DSM 2079 / JCM 5827 / CCUG 10774 / NCTC 10582 / VPI-5482 / E50) OX=226186 GN=susB PE=1 SV=1 |
| D7CFN7 | 5.72e-40 | 50 | 572 | 65 | 574 | Probable retaining alpha-galactosidase OS=Streptomyces bingchenggensis (strain BCW-1) OX=749414 GN=SBI_01652 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
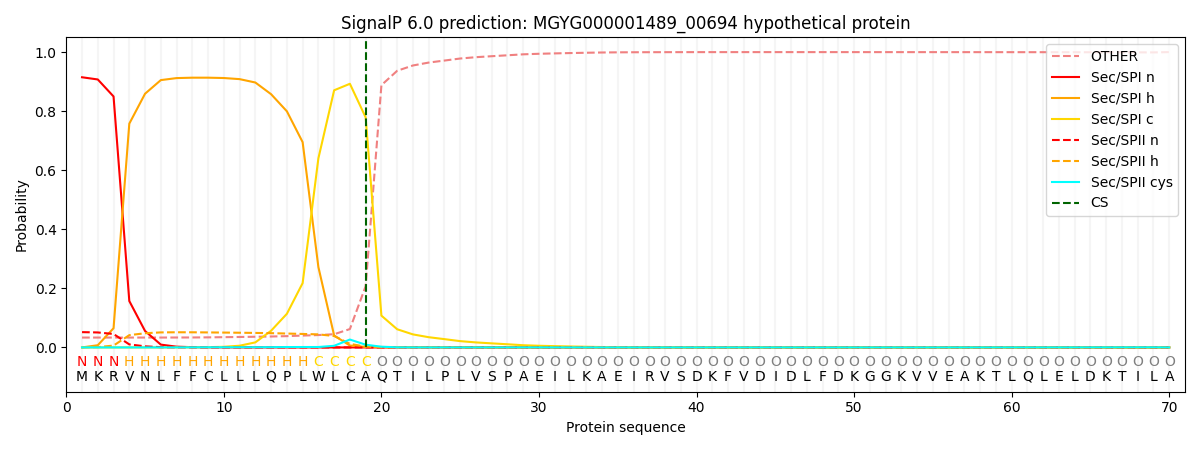
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.038880 | 0.906139 | 0.053985 | 0.000344 | 0.000298 | 0.000326 |
