You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001489_01772
You are here: Home > Sequence: MGYG000001489_01772
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Parabacteroides goldsteinii | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Tannerellaceae; Parabacteroides; Parabacteroides goldsteinii | |||||||||||
| CAZyme ID | MGYG000001489_01772 | |||||||||||
| CAZy Family | GH29 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 2341574; End: 2342971 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH29 | 28 | 358 | 5.8e-60 | 0.8641618497109826 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3669 | AfuC | 5.30e-21 | 26 | 458 | 9 | 430 | Alpha-L-fucosidase [Carbohydrate transport and metabolism]. |
| pfam01120 | Alpha_L_fucos | 2.60e-12 | 72 | 356 | 79 | 326 | Alpha-L-fucosidase. |
| smart00812 | Alpha_L_fucos | 1.18e-10 | 72 | 248 | 76 | 254 | Alpha-L-fucosidase. O-Glycosyl hydrolases (EC 3.2.1.-) are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycosyl hydrolases, based on sequence similarity, has led to the definition of 85 different families. This classification is available on the CAZy (CArbohydrate-Active EnZymes) web site. Because the fold of proteins is better conserved than their sequences, some of the families can be grouped in 'clans'. Family 29 encompasses alpha-L-fucosidases, which is a lysosomal enzyme responsible for hydrolyzing the alpha-1,6-linked fucose joined to the reducing-end N-acetylglucosamine of the carbohydrate moieties of glycoproteins. Deficiency of alpha-L-fucosidase results in the lysosomal storage disease fucosidosis. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUU07432.1 | 9.56e-290 | 1 | 461 | 1 | 460 |
| QRP56810.1 | 3.71e-287 | 1 | 461 | 1 | 460 |
| QQT77946.1 | 3.71e-287 | 1 | 461 | 1 | 460 |
| ASM65515.1 | 3.71e-287 | 1 | 461 | 1 | 460 |
| QKH84971.1 | 3.04e-286 | 6 | 460 | 6 | 459 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3GZA_A | 4.01e-279 | 20 | 461 | 2 | 443 | Crystalstructure of putative alpha-L-fucosidase (NP_812709.1) from BACTEROIDES THETAIOTAOMICRON VPI-5482 at 1.60 A resolution [Bacteroides thetaiotaomicron VPI-5482],3GZA_B Crystal structure of putative alpha-L-fucosidase (NP_812709.1) from BACTEROIDES THETAIOTAOMICRON VPI-5482 at 1.60 A resolution [Bacteroides thetaiotaomicron VPI-5482] |
| 5K9H_A | 2.16e-69 | 16 | 463 | 27 | 468 | Crystalstructure of a glycoside hydrolase 29 family member from an unknown rumen bacterium [unidentified] |
| 3EYP_A | 2.13e-62 | 28 | 465 | 10 | 461 | Crystalstructure of putative alpha-L-fucosidase from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron],3EYP_B Crystal structure of putative alpha-L-fucosidase from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron],4OUE_A Crystal structure of an a-L-Fucosidase GH29 from Bacteroides thetaiotaomicron (BT2192) in complex with IPTG [Bacteroides thetaiotaomicron VPI-5482],4OUE_B Crystal structure of an a-L-Fucosidase GH29 from Bacteroides thetaiotaomicron (BT2192) in complex with IPTG [Bacteroides thetaiotaomicron VPI-5482],4OZO_A Crystal structure of an a-L-fucosidase GH29 from Bacteroides thetaiotaomicron (BT2192) in complex with oNPTG [Bacteroides thetaiotaomicron VPI-5482],4OZO_B Crystal structure of an a-L-fucosidase GH29 from Bacteroides thetaiotaomicron (BT2192) in complex with oNPTG [Bacteroides thetaiotaomicron VPI-5482] |
| 4ZRX_A | 1.87e-60 | 28 | 445 | 32 | 445 | Crystalstructure of a putative alpha-L-fucosidase (BACOVA_04357) from Bacteroides ovatus ATCC 8483 at 1.59 A resolution [Bacteroides ovatus ATCC 8483] |
| 3UES_A | 1.44e-48 | 27 | 452 | 18 | 470 | Crystalstructure of alpha-1,3/4-fucosidase from Bifidobacterium longum subsp. infantis complexed with deoxyfuconojirimycin [Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 = DSM 20088],3UES_B Crystal structure of alpha-1,3/4-fucosidase from Bifidobacterium longum subsp. infantis complexed with deoxyfuconojirimycin [Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 = DSM 20088] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q7XUR3 | 3.59e-60 | 19 | 458 | 24 | 476 | Putative alpha-L-fucosidase 1 OS=Oryza sativa subsp. japonica OX=39947 GN=Os04g0560400 PE=3 SV=2 |
| Q8GW72 | 4.26e-58 | 25 | 458 | 35 | 477 | Alpha-L-fucosidase 1 OS=Arabidopsis thaliana OX=3702 GN=FUC1 PE=1 SV=2 |
| Q6AYS4 | 5.24e-06 | 67 | 355 | 93 | 349 | Plasma alpha-L-fucosidase OS=Rattus norvegicus OX=10116 GN=Fuca2 PE=2 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
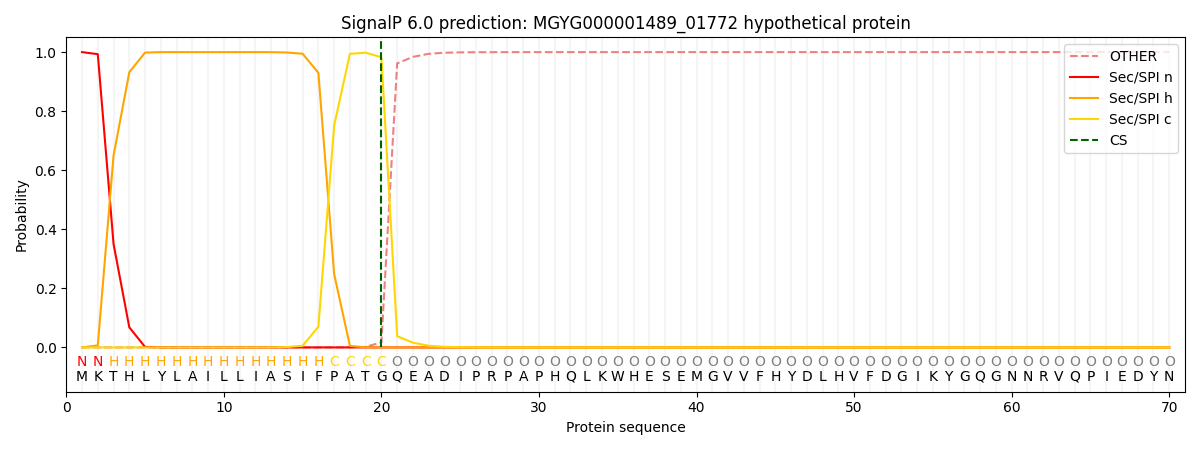
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000196 | 0.999187 | 0.000172 | 0.000146 | 0.000136 | 0.000127 |
