You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001493_03134
You are here: Home > Sequence: MGYG000001493_03134
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Enterocloster bolteae | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Lachnospirales; Lachnospiraceae; Enterocloster; Enterocloster bolteae | |||||||||||
| CAZyme ID | MGYG000001493_03134 | |||||||||||
| CAZy Family | GH25 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 403134; End: 404939 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH25 | 103 | 272 | 8.3e-46 | 0.9943502824858758 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd06414 | GH25_LytC-like | 6.22e-83 | 100 | 282 | 1 | 191 | The LytC lysozyme of Streptococcus pneumoniae is a bacterial cell wall hydrolase that cleaves the beta1-4-glycosydic bond located between the N-acetylmuramoyl-N-glucosaminyl residues of the cell wall polysaccharide chains. LytC is composed of a C-terminal glycosyl hydrolase family 25 (GH25) domain and an N-terminal choline-binding module (CBM) consisting of eleven homologous repeats that specifically recognizes the choline residues of pneumococcal lipoteichoic and teichoic acids. This domain arrangement is the reverse of the major pneumococcal autolysin, LytA, and the CPL-1-like lytic enzymes of the pneumococcal bacteriophages, in which the CBM (consisting of six repeats) is at the C-terminus. This model represents the C-terminal catalytic domain of the LytC-like enzymes. |
| NF033930 | pneumo_PspA | 1.94e-66 | 290 | 492 | 441 | 643 | pneumococcal surface protein A. The pneumococcal surface protein proteins, found in Streptococcus pneumoniae, are repetitive, with patterns of localized high sequence identity across pairs of proteins given different specific names that recombination may be presumed. This protein, PspA, has an N-terminal region that lacks a cross-wall-targeting YSIRK type extended signal peptide, in contrast to the closely related choline-binding protein CbpA which has a similar C-terminus but a YSIRK-containing region at the N-terminus. |
| NF033930 | pneumo_PspA | 2.53e-62 | 311 | 551 | 442 | 654 | pneumococcal surface protein A. The pneumococcal surface protein proteins, found in Streptococcus pneumoniae, are repetitive, with patterns of localized high sequence identity across pairs of proteins given different specific names that recombination may be presumed. This protein, PspA, has an N-terminal region that lacks a cross-wall-targeting YSIRK type extended signal peptide, in contrast to the closely related choline-binding protein CbpA which has a similar C-terminus but a YSIRK-containing region at the N-terminus. |
| NF033838 | PspC_subgroup_1 | 1.98e-61 | 311 | 532 | 485 | 683 | pneumococcal surface protein PspC, choline-binding form. The pneumococcal surface protein PspC, as described in Streptococcus pneumoniae, is a repetitive and highly variable protein, recognized by a conserved N-terminal domain and also by genomic location. This form, subgroup 1, has variable numbers of a choline-binding repeat in the C-terminal region, and is also known as choline-binding protein A. The other form, subgroup 2, is anchored covalently after cleavage by sortase at a C-terminal LPXTG site. |
| NF033838 | PspC_subgroup_1 | 2.83e-60 | 290 | 492 | 484 | 666 | pneumococcal surface protein PspC, choline-binding form. The pneumococcal surface protein PspC, as described in Streptococcus pneumoniae, is a repetitive and highly variable protein, recognized by a conserved N-terminal domain and also by genomic location. This form, subgroup 1, has variable numbers of a choline-binding repeat in the C-terminal region, and is also known as choline-binding protein A. The other form, subgroup 2, is anchored covalently after cleavage by sortase at a C-terminal LPXTG site. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QRP37172.1 | 1.29e-251 | 1 | 601 | 1 | 601 |
| ASN98018.1 | 1.29e-251 | 1 | 601 | 1 | 601 |
| QJU23033.1 | 2.48e-244 | 1 | 601 | 1 | 601 |
| QQR03194.1 | 1.44e-226 | 1 | 600 | 1 | 540 |
| ANU47907.1 | 1.44e-226 | 1 | 600 | 1 | 540 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4KRU_A | 1.64e-15 | 101 | 279 | 21 | 205 | X-raystructure of catalytic domain of endolysin from clostridium perfringens phage phiSM101 [Clostridium phage phiSM101] |
| 4KRT_A | 8.67e-15 | 101 | 279 | 21 | 205 | X-raystructure of endolysin from clostridium perfringens phage phiSM101 [Clostridium phage phiSM101],4KRT_B X-ray structure of endolysin from clostridium perfringens phage phiSM101 [Clostridium phage phiSM101] |
| 5A6S_A | 1.60e-13 | 89 | 291 | 15 | 211 | Crystalstructure of the CTP1L endolysin reveals how its activity is regulated by a secondary translation product [Clostridium phage phiCTP1] |
| 1JFX_A | 2.39e-10 | 101 | 277 | 6 | 198 | Crystalstructure of the bacterial lysozyme from Streptomyces coelicolor at 1.65 A resolution [Streptomyces coelicolor] |
| 3HMC_A | 6.02e-09 | 103 | 279 | 8 | 177 | Endolysinfrom Bacillus anthracis [Bacillus anthracis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8X7H0 | 1.84e-14 | 102 | 279 | 69 | 252 | Uncharacterized protein YegX OS=Escherichia coli O157:H7 OX=83334 GN=yegX PE=3 SV=2 |
| P76421 | 1.10e-13 | 102 | 279 | 69 | 252 | Uncharacterized protein YegX OS=Escherichia coli (strain K12) OX=83333 GN=yegX PE=3 SV=2 |
| Q8FFY2 | 1.49e-13 | 102 | 279 | 69 | 252 | Uncharacterized protein YegX OS=Escherichia coli O6:H1 (strain CFT073 / ATCC 700928 / UPEC) OX=199310 GN=yegX PE=3 SV=2 |
| P26836 | 2.47e-13 | 91 | 284 | 2 | 198 | Probable autolytic lysozyme OS=Clostridium perfringens (strain 13 / Type A) OX=195102 GN=lyc PE=3 SV=2 |
| P34020 | 7.18e-11 | 101 | 279 | 2 | 178 | Autolytic lysozyme OS=Clostridium acetobutylicum (strain ATCC 824 / DSM 792 / JCM 1419 / LMG 5710 / VKM B-1787) OX=272562 GN=lyc PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
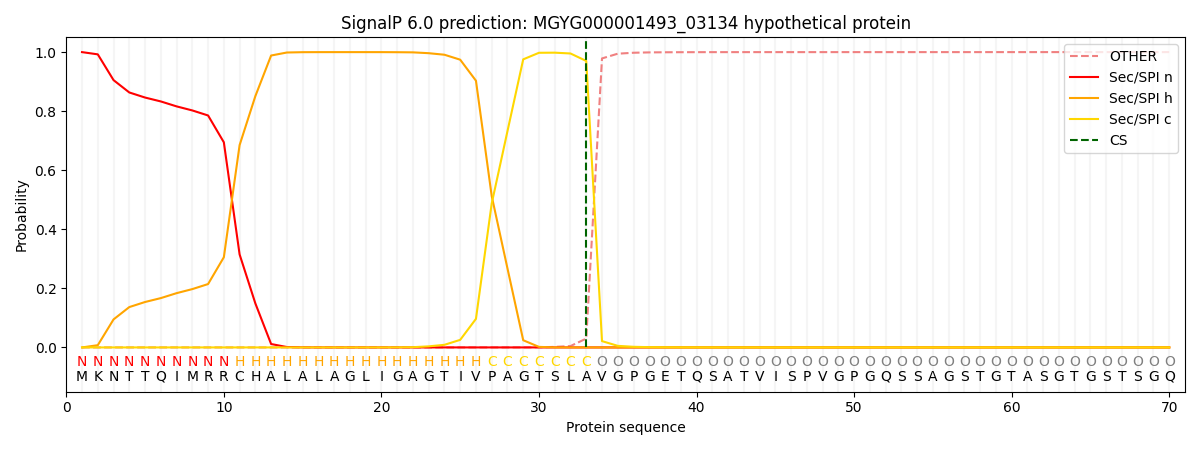
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000362 | 0.998827 | 0.000181 | 0.000247 | 0.000186 | 0.000156 |

