You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001512_01818
You are here: Home > Sequence: MGYG000001512_01818
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Coprobacter secundus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Coprobacteraceae; Coprobacter; Coprobacter secundus | |||||||||||
| CAZyme ID | MGYG000001512_01818 | |||||||||||
| CAZy Family | PL8 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 1043450; End: 1046608 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL8 | 547 | 805 | 2.7e-50 | 0.97165991902834 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd01083 | GAG_Lyase | 8.72e-46 | 262 | 897 | 72 | 693 | Glycosaminoglycan (GAG) polysaccharide lyase family. This family consists of a group of secreted bacterial lyase enzymes capable of acting on glycosaminoglycans, such as hyaluronan and chondroitin, in the extracellular matrix of host tissues, contributing to the invasive capacity of the pathogen. These are broad-specificity glycosaminoglycan lyases which recognize uronyl residues in polysaccharides and cleave their glycosidic bonds via a beta-elimination reaction to form a double bond between C-4 and C-5 of the non-reducing terminal uronyl residues of released products. Substrates include chondroitin, chondroitin 4-sulfate, chondroitin 6-sulfate, and hyaluronic acid. Family members include chondroitin AC lyase, chondroitin abc lyase, xanthan lyase, and hyalurate lyase. |
| pfam09093 | Lyase_catalyt | 7.85e-26 | 200 | 518 | 7 | 351 | Lyase, catalytic. Members of this family are predominantly found in chondroitin ABC lyase I, and adopt a helical structure, with fifteen alpha-helices which are at least two turns long and several short helical turns. The bulk of the domain is formed by ten alpha-helices forming five hairpin-like pairs and arranged into an incomplete toroid, the (alpha/alpha)5 fold. Additionally, two long and two short alpha-helices at the N-terminus of the domain wrap around the toroid. At the C-terminal end of the toroid there is one additional short alpha-helix. This domain is required for degradation of polysaccharides containing 1,4-beta-D-hexosaminyl and 1,3-beta-D-glucoronosyl or 1,3-alpha-L-iduronosyl linkages to disaccharides containing 4-deoxy-beta-D-gluc-4-enuronosyl groups. |
| pfam02278 | Lyase_8 | 4.02e-17 | 537 | 815 | 4 | 252 | Polysaccharide lyase family 8, super-sandwich domain. This family consists of a group of secreted bacterial lyase enzymes EC:4.2.2.1 capable of acting on hyaluronan and chondroitin in the extracellular matrix of host tissues, contributing to the invasive capacity of the pathogen. |
| pfam09092 | Lyase_N | 8.32e-11 | 21 | 115 | 4 | 110 | Lyase, N terminal. Members of this family are predominantly found in chondroitin ABC lyase I, and adopt a jelly-roll fold topology consisting of a two-layered bent beta-sheet sandwich with one short alpha-helix. The convex beta sheet is composed of five antiparallel strands, whilst the concave beta-sheet contains five antiparallel beta-strands with a loop between two consecutive strands folding back onto the concave surface. This domain is required for binding of the protein to long glycosaminoglycan chains. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BCI63816.1 | 1.25e-252 | 1 | 1051 | 1 | 1060 |
| BCI64222.1 | 1.02e-244 | 22 | 1051 | 25 | 1069 |
| QJB31525.1 | 1.69e-144 | 1 | 943 | 1 | 949 |
| QJB38007.1 | 1.75e-143 | 1 | 943 | 1 | 949 |
| QEK51405.1 | 2.48e-137 | 1 | 952 | 1 | 947 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 7EIP_A | 1.40e-44 | 39 | 942 | 61 | 1002 | ChainA, Chondroitin sulfate ABC endolyase [Proteus vulgaris],7EIQ_A Chain A, Chondroitin sulfate ABC endolyase [Proteus vulgaris],7EIR_A Chain A, Chondroitin sulfate ABC endolyase [Proteus vulgaris],7EIS_A Chain A, Chondroitin sulfate ABC endolyase [Proteus vulgaris] |
| 1HN0_A | 1.85e-44 | 39 | 942 | 61 | 1002 | CRYSTALSTRUCTURE OF CHONDROITIN ABC LYASE I FROM PROTEUS VULGARIS AT 1.9 ANGSTROMS RESOLUTION [Proteus vulgaris] |
| 2Q1F_A | 5.18e-36 | 22 | 952 | 23 | 1005 | Crystalstructure of chondroitin sulfate lyase abc from bacteroides thetaiotaomicron wal2926 [Bacteroides thetaiotaomicron],2Q1F_B Crystal structure of chondroitin sulfate lyase abc from bacteroides thetaiotaomicron wal2926 [Bacteroides thetaiotaomicron] |
| 1J0M_A | 1.96e-07 | 666 | 823 | 470 | 632 | CrystalStructure of Bacillus sp. GL1 Xanthan Lyase that Acts on Side Chains of Xanthan [Bacillus sp. GL1],1J0N_A Crystal Structure of Bacillus sp. GL1 Xanthan Lyase that Acts on Side Chains of Xanthan [Bacillus sp. GL1] |
| 1X1H_A | 1.96e-07 | 666 | 823 | 470 | 632 | ChainA, xanthan lyase [Bacillus sp. GL1],1X1I_A Chain A, xanthan lyase [Bacillus sp. GL1],1X1J_A Chain A, xanthan lyase [Bacillus sp. GL1] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P59807 | 7.68e-44 | 39 | 942 | 61 | 1002 | Chondroitin sulfate ABC endolyase OS=Proteus vulgaris OX=585 PE=1 SV=2 |
| C7S340 | 2.15e-43 | 21 | 936 | 12 | 966 | Chondroitin sulfate ABC exolyase (Fragment) OS=Proteus vulgaris OX=585 GN=ChABCII PE=1 SV=1 |
| C5G6D7 | 2.45e-40 | 19 | 952 | 20 | 1005 | Chondroitin sulfate ABC exolyase OS=Bacteroides thetaiotaomicron OX=818 GN=chonabc PE=1 SV=2 |
| Q8A2I1 | 4.27e-40 | 19 | 899 | 20 | 933 | Chondroitin sulfate ABC exolyase OS=Bacteroides thetaiotaomicron (strain ATCC 29148 / DSM 2079 / JCM 5827 / CCUG 10774 / NCTC 10582 / VPI-5482 / E50) OX=226186 GN=chonabc PE=1 SV=1 |
| Q9AQS0 | 1.16e-06 | 666 | 823 | 495 | 657 | Xanthan lyase OS=Bacillus sp. (strain GL1) OX=84635 GN=xly PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
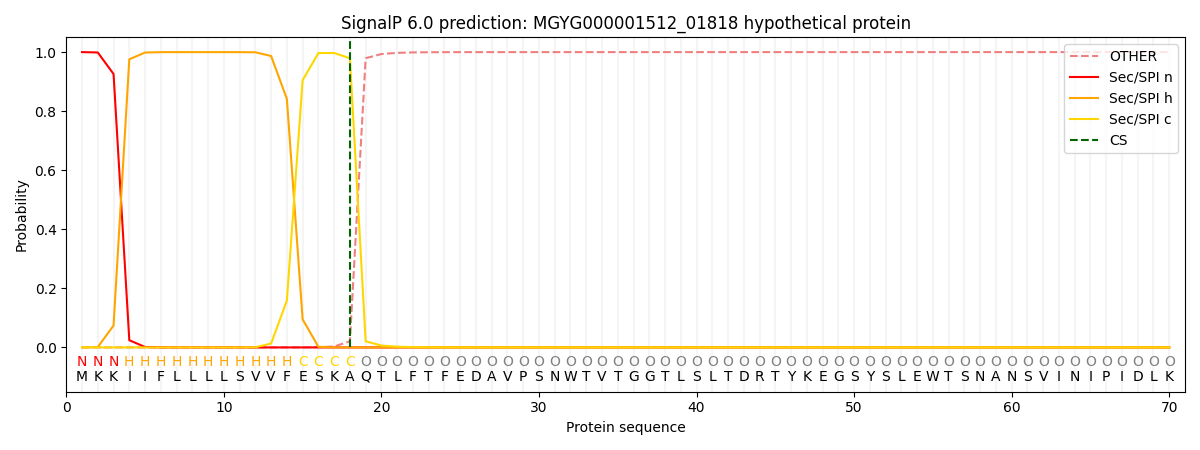
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000218 | 0.999145 | 0.000174 | 0.000156 | 0.000147 | 0.000138 |
