You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001540_00635
You are here: Home > Sequence: MGYG000001540_00635
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Corynebacterium provencense | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Actinobacteriota; Actinomycetia; Mycobacteriales; Mycobacteriaceae; Corynebacterium; Corynebacterium provencense | |||||||||||
| CAZyme ID | MGYG000001540_00635 | |||||||||||
| CAZy Family | GH25 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 703480; End: 704826 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH25 | 158 | 353 | 7.2e-30 | 0.9887005649717514 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam01551 | Peptidase_M23 | 4.16e-29 | 23 | 132 | 1 | 96 | Peptidase family M23. Members of this family are zinc metallopeptidases with a range of specificities. The peptidase family M23 is included in this family, these are Gly-Gly endopeptidases. Peptidase family M23 are also endopeptidases. This family also includes some bacterial lipoproteins for which no proteolytic activity has been demonstrated. This family also includes leukocyte cell-derived chemotaxin 2 (LECT2) proteins. LECT2 is a liver-specific protein which is thought to be linked to hepatocyte growth although the exact function of this protein is unknown. |
| COG0739 | NlpD | 4.69e-26 | 5 | 143 | 137 | 271 | Murein DD-endopeptidase MepM and murein hydrolase activator NlpD, contain LysM domain [Cell wall/membrane/envelope biogenesis]. |
| cd12797 | M23_peptidase | 1.62e-25 | 25 | 118 | 1 | 85 | M23 family metallopeptidase, also known as beta-lytic metallopeptidase, and similar proteins. This model describes the metallopeptidase M23 family, which includes beta-lytic metallopeptidase and lysostaphin. Members of this family are zinc endopeptidases that lyse bacterial cell wall peptidoglycans; they cleave either the N-acylmuramoyl-Ala bond between the cell wall peptidoglycan and the cross-linking peptide (e.g. beta-lytic endopeptidase) or a bond within the cross-linking peptide (e.g. stapholysin, and lysostaphin). Beta-lytic metallopeptidase, formerly known as beta-lytic protease, has a preference for cleavage of Gly-X bonds and favors hydrophobic or apolar residues on either side. It inhibits growth of sensitive organisms and may potentially serve as an antimicrobial agent. Lysostaphin, produced by Staphylococcus genus, cleaves pentaglycine cross-bridges of cell wall peptidoglycan, acting as autolysins to maintain cell wall metabolism or as toxins and weapons against competing strains. Staphylolysin (also known as LasA) is implicated in a range of processes related to Pseudomonas virulence, including stimulating shedding of the ectodomain of cell surface heparan sulphate proteoglycan syndecan-1, and elastin degradation in connective tissue. Its active site is less constricted and contains a five-coordinate zinc ion with trigonal bipyramidal geometry and two metal-bound water molecules, possibly contributing to its activity against a wider range of substrates than those used by related lytic enzymes, consistent with its multiple roles in Pseudomonas virulence. The family includes members that do not appear to have the conserved zinc-binding site and might be lipoproteins lacking proteolytic activity. |
| cd00599 | GH25_muramidase | 1.06e-22 | 157 | 361 | 2 | 186 | Endo-N-acetylmuramidases (muramidases) are lysozymes (also referred to as peptidoglycan hydrolases) that degrade bacterial cell walls by catalyzing the hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues. This family of muramidases contains a glycosyl hydrolase family 25 (GH25) catalytic domain and is found in bacteria, fungi, slime molds, round worms, protozoans and bacteriophages. The bacteriophage members are referred to as endolysins which are involved in lysing the host cell at the end of the replication cycle to allow release of mature phage particles. Endolysins are typically modular enzymes consisting of a catalytically active domain that hydrolyzes the peptidoglycan cell wall and a cell wall-binding domain that anchors the protein to the cell wall. Endolysins generally have narrow substrate specificities with either intra-species or intra-genus bacteriolytic activity. |
| cd06414 | GH25_LytC-like | 4.19e-16 | 155 | 359 | 1 | 188 | The LytC lysozyme of Streptococcus pneumoniae is a bacterial cell wall hydrolase that cleaves the beta1-4-glycosydic bond located between the N-acetylmuramoyl-N-glucosaminyl residues of the cell wall polysaccharide chains. LytC is composed of a C-terminal glycosyl hydrolase family 25 (GH25) domain and an N-terminal choline-binding module (CBM) consisting of eleven homologous repeats that specifically recognizes the choline residues of pneumococcal lipoteichoic and teichoic acids. This domain arrangement is the reverse of the major pneumococcal autolysin, LytA, and the CPL-1-like lytic enzymes of the pneumococcal bacteriophages, in which the CBM (consisting of six repeats) is at the C-terminus. This model represents the C-terminal catalytic domain of the LytC-like enzymes. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AHI04498.1 | 2.68e-195 | 1 | 374 | 3 | 372 |
| QQB45370.1 | 5.02e-195 | 1 | 446 | 1 | 457 |
| ARM68445.1 | 6.15e-189 | 1 | 389 | 1 | 401 |
| AHW64888.1 | 6.59e-148 | 20 | 416 | 2 | 399 |
| QDF20033.1 | 1.57e-147 | 5 | 440 | 6 | 444 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5J1L_A | 9.19e-13 | 25 | 118 | 65 | 151 | Crystalstructure of Csd1-Csd2 dimer I [Helicobacter pylori 26695],5J1L_C Crystal structure of Csd1-Csd2 dimer I [Helicobacter pylori 26695],5J1M_A Crystal structure of Csd1-Csd2 dimer II [Helicobacter pylori 26695],5J1M_C Crystal structure of Csd1-Csd2 dimer II [Helicobacter pylori 26695] |
| 6UE4_A | 3.87e-12 | 25 | 118 | 266 | 350 | ShyAEndopeptidase from Vibrio cholerae (Closed form) [Vibrio cholerae O1 biovar El Tor str. N16961],6UE4_B ShyA Endopeptidase from Vibrio cholerae (Closed form) [Vibrio cholerae O1 biovar El Tor str. N16961] |
| 6U2A_A | 3.91e-12 | 25 | 118 | 263 | 347 | ShyAendopeptidase from Vibrio cholera (open form) [Vibrio cholerae] |
| 6SMK_A | 6.27e-12 | 14 | 132 | 15 | 126 | Crystalstructure of catalytic domain A109H mutant of prophage-encoded M23 protein EnpA from Enterococcus faecalis. [Enterococcus faecalis V583],6SMK_B Crystal structure of catalytic domain A109H mutant of prophage-encoded M23 protein EnpA from Enterococcus faecalis. [Enterococcus faecalis V583],6SMK_C Crystal structure of catalytic domain A109H mutant of prophage-encoded M23 protein EnpA from Enterococcus faecalis. [Enterococcus faecalis V583],6SMK_D Crystal structure of catalytic domain A109H mutant of prophage-encoded M23 protein EnpA from Enterococcus faecalis. [Enterococcus faecalis V583],6SMK_E Crystal structure of catalytic domain A109H mutant of prophage-encoded M23 protein EnpA from Enterococcus faecalis. [Enterococcus faecalis V583] |
| 6JMX_A | 1.16e-10 | 17 | 137 | 141 | 247 | ChainA, Peptidase M23 [Campylobacter jejuni],6JMY_A Chain A, Peptidase M23 [Campylobacter jejuni],6KV1_A Chain A, Peptidase M23 [Campylobacter jejuni] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q89AI9 | 3.36e-12 | 19 | 119 | 245 | 336 | Uncharacterized metalloprotease bbp_296 OS=Buchnera aphidicola subsp. Baizongia pistaciae (strain Bp) OX=224915 GN=bbp_296 PE=3 SV=1 |
| P9WKW8 | 1.62e-10 | 5 | 150 | 248 | 392 | Uncharacterized protein MT3894 OS=Mycobacterium tuberculosis (strain CDC 1551 / Oshkosh) OX=83331 GN=MT3894 PE=3 SV=1 |
| P0A5H2 | 1.62e-10 | 5 | 150 | 248 | 392 | Uncharacterized protein Mb3815c OS=Mycobacterium bovis (strain ATCC BAA-935 / AF2122/97) OX=233413 GN=BQ2027_MB3815C PE=3 SV=1 |
| P9WKW9 | 1.62e-10 | 5 | 150 | 248 | 392 | Uncharacterized protein Rv3786c OS=Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) OX=83332 GN=Rv3786c PE=1 SV=1 |
| Q8K9M4 | 3.93e-10 | 25 | 119 | 291 | 376 | Uncharacterized metalloprotease BUsg_310 OS=Buchnera aphidicola subsp. Schizaphis graminum (strain Sg) OX=198804 GN=BUsg_310 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
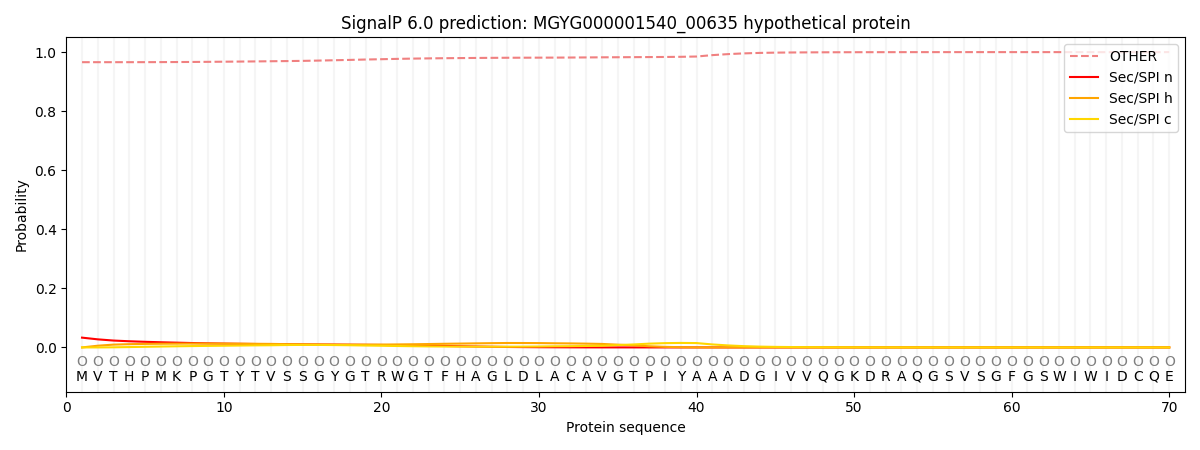
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.967788 | 0.030991 | 0.001066 | 0.000046 | 0.000032 | 0.000080 |
