You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001542_00637
You are here: Home > Sequence: MGYG000001542_00637
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
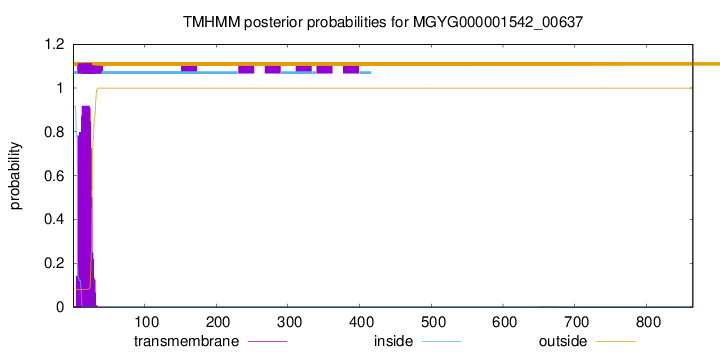
TMHMM annotations
Basic Information help
| Species | Paenibacillus_A sp900069005 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Paenibacillales; Paenibacillaceae; Paenibacillus_A; Paenibacillus_A sp900069005 | |||||||||||
| CAZyme ID | MGYG000001542_00637 | |||||||||||
| CAZy Family | CBM35 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 79885; End: 82482 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CBM35 | 45 | 163 | 1.1e-22 | 0.9831932773109243 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd04081 | CBM35_galactosidase-like | 1.59e-36 | 44 | 164 | 2 | 125 | Carbohydrate Binding Module family 35 (CBM35); appended mainly to enzymes that bind alpha-D-galactose (CBM35-Gal), including glycoside hydrolase (GH) families GH27 and GH43. This family includes carbohydrate binding module family 35 (CBM35); these are non-catalytic carbohydrate binding domains that are appended mainly to enzymes that bind alpha-D-galactose (CBM35-Gal), including glycoside hydrolase (GH) families GH27 and GH43. Examples of proteins which contain CBM35s belonging to this family includes the CBM35 of an exo-beta-1,3-galactanase from Phanerochaete chrysosporium 9 (Pc1,3Gal43A) which is appended to a GH43 domain, and the CBM35 domain of two bifunctional proteins with beta-L-arabinopyranosidase/alpha-D-galactopyranosidase activities from Fusarium oxysporum 12S, Foap1 and Foap2 (Fo/AP1 and Fo/AP2), that are appended to GH27 domains. CBM35s are unique in that they display conserved specificity through extensive sequence similarity but divergent function through their appended catalytic modules. They are known to bind alpha-D-galactose (Gal), mannan (Man), xylan, glucuronic acid (GlcA), a beta-polymer of mannose, and possibly glucans, forming four subfamilies based on general ligand specificities (galacto, urono, manno, and gluco configurations). Some CBM35s bind their ligands in a calcium-dependent manner. In contrast to most CBMs that are generally rigid proteins, CBM35 undergoes significant conformational change upon ligand binding. GH43 includes beta-xylosidases and beta-xylanases, using aryl-glycosides as substrates, while family GH27 includes alpha-galactosidases, alpha-N-acetylgalactosaminidases, and isomaltodextranases. |
| cd04083 | CBM35_Lmo2446-like | 4.18e-13 | 43 | 164 | 1 | 125 | Carbohydrate Binding Module 35 (CBM35) domains similar to Lmo2446. This family includes carbohydrate binding module 35 (CBM35) domains that are appended to several carbohydrate binding enzymes. Some CBM35 domains belonging to this family are appended to glycoside hydrolase (GH) family domains, including glycoside hydrolase family 31 (GH31), for example the CBM35 domain of Lmo2446, an uncharacterized protein from Listeria monocytogenes EGD-e. These CBM35s are non-catalytic carbohydrate binding domains that facilitate the strong binding of the GH catalytic modules with their dedicated, insoluble substrates. GH31 has a wide range of hydrolytic activities such as alpha-glucosidase, alpha-xylosidase, 6-alpha-glucosyltransferase, or alpha-1,4-glucan lyase, cleaving a terminal carbohydrate moiety from a substrate that may be a starch or a glycoprotein. Most characterized GH31 enzymes are alpha-glucosidases. |
| cd14792 | GH27 | 9.65e-13 | 474 | 771 | 2 | 268 | glycosyl hydrolase family 27 (GH27). GH27 enzymes occur in eukaryotes, prokaryotes, and archaea with a wide range of hydrolytic activities, including alpha-glucosidase (glucoamylase and sucrase-isomaltase), alpha-N-acetylgalactosaminidase, and 3-alpha-isomalto-dextranase. All GH27 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH27 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| cd14791 | GH36 | 8.10e-12 | 474 | 755 | 3 | 286 | glycosyl hydrolase family 36 (GH36). GH36 enzymes occur in prokaryotes, eukaryotes, and archaea with a wide range of hydrolytic activities, including alpha-galactosidase, alpha-N-acetylgalactosaminidase, stachyose synthase, and raffinose synthase. All GH36 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. GH36 members are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
| cd04082 | CBM35_pectate_lyase-like | 1.17e-11 | 78 | 162 | 35 | 122 | Carbohydrate Binding Module family 35 (CBM35), pectate lyase-like; appended mainly to enzymes that bind mannan (Man), xylan, glucuronic acid (GlcA) and possibly glucans. This family includes carbohydrate binding module family 35 (CBM35) domains that are non-catalytic carbohydrate binding domains that are appended mainly to enzymes that bind mannan (Man), xylan, glucuronic acid (GlcA) and possibly glucans. Included in this family are CBM35s of pectate lyases, including pectate lyase 10A from Cellvibrio japonicas, these enzymes release delta-4,5-anhydrogalaturonic acid (delta4,5-GalA) from pectin, thus identifying a signature molecule for plant cell wall degradation. CBM35s are unique in that they display conserved specificity through extensive sequence similarity but divergent function through their appended catalytic modules. They are known to bind alpha-D-galactose (Gal), mannan (Man), xylan, glucuronic acid (GlcA), a beta-polymer of mannose, and possibly glucans, forming four subfamilies based on general ligand specificities (galacto, urono, manno, and gluco configurations). In contrast to most CBMs that are generally rigid proteins, CBM35 undergoes significant conformational change upon ligand binding. Some CBM35s bind their ligands in a calcium-dependent manner, especially those binding uronic acids. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AWP26688.1 | 0.0 | 41 | 862 | 45 | 866 |
| AYB43259.1 | 0.0 | 1 | 862 | 1 | 865 |
| ANA78555.1 | 0.0 | 1 | 862 | 1 | 866 |
| AVV57528.1 | 0.0 | 1 | 862 | 1 | 866 |
| ACX66630.1 | 0.0 | 1 | 862 | 1 | 865 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5AWO_A | 9.39e-07 | 543 | 862 | 120 | 465 | Arthrobacterglobiformis T6 isomalto-dextranse [Arthrobacter globiformis],5AWP_A Arthrobacter globiformis T6 isomalto-dextranase complexed with isomaltose [Arthrobacter globiformis],5AWQ_A Arthrobacter globiformis T6 isomalto-dextranse complexed with panose [Arthrobacter globiformis] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q44052 | 3.05e-06 | 543 | 862 | 146 | 491 | Isomalto-dextranase OS=Arthrobacter globiformis OX=1665 GN=imd PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
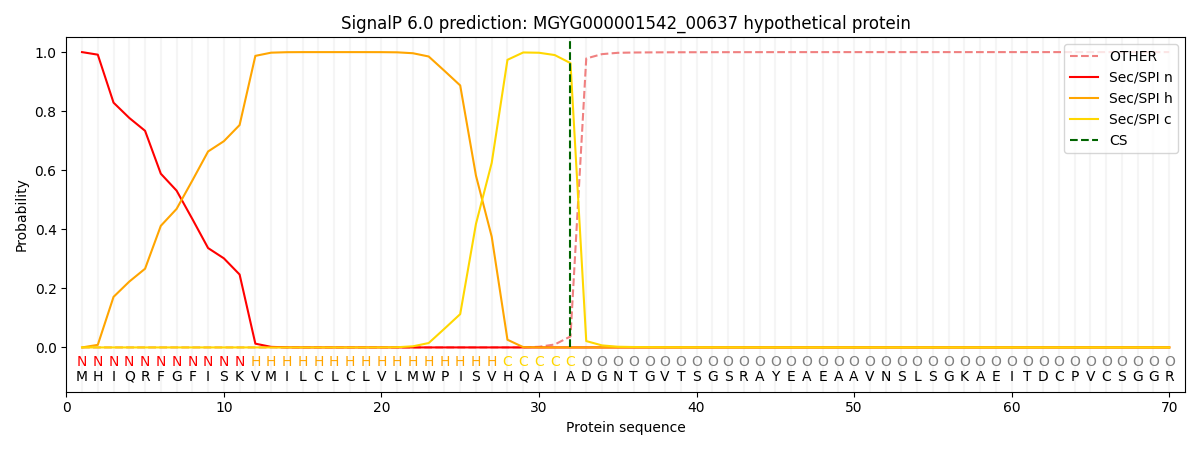
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000356 | 0.998984 | 0.000178 | 0.000172 | 0.000150 | 0.000151 |