You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001542_02066
You are here: Home > Sequence: MGYG000001542_02066
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Paenibacillus_A sp900069005 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Paenibacillales; Paenibacillaceae; Paenibacillus_A; Paenibacillus_A sp900069005 | |||||||||||
| CAZyme ID | MGYG000001542_02066 | |||||||||||
| CAZy Family | GH39 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 77147; End: 80476 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CBM9 | 943 | 1091 | 8.6e-19 | 0.7802197802197802 |
| GH39 | 354 | 652 | 1.3e-17 | 0.7053364269141531 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd09621 | CBM9_like_5 | 1.42e-43 | 927 | 1083 | 1 | 165 | DOMON-like type 9 carbohydrate binding module. Family 9 carbohydrate-binding modules (CBM9) play a role in the microbial degradation of cellulose and hemicellulose (materials found in plants). The domain has previously been called cellulose-binding domain. The polysaccharide binding sites of CBMs with available 3D structure have been found to be either flat surfaces with interactions formed by predominantly aromatic residues (tryptophan and tyrosine), or extended shallow grooves. CBM9 domains found in this uncharacterized heterogeneous subfamily are often located at the C-terminus of longer proteins and may co-occur with various other functional domains such as glycosyl hydrolases. The CBM9 module in these architectures may be involved in binding to carbohydrates. |
| cd09619 | CBM9_like_4 | 3.20e-14 | 926 | 1083 | 15 | 166 | DOMON-like type 9 carbohydrate binding module. Family 9 carbohydrate-binding modules (CBM9) play a role in the microbial degradation of cellulose and hemicellulose (materials found in plants). The domain has previously been called cellulose-binding domain. The polysaccharide binding sites of CBMs with available 3D structure have been found to be either flat surfaces with interactions formed by predominantly aromatic residues (tryptophan and tyrosine), or extended shallow grooves. CBM9 domains found in this uncharacterized heterogeneous subfamily are often located at the C-terminus of longer proteins and may co-occur with various other domains. |
| cd00241 | DOMON_like | 2.85e-13 | 940 | 1083 | 10 | 153 | Domon-like ligand-binding domains. DOMON-like domains can be found in all three kindgoms of life and are a diverse group of ligand binding domains that have been shown to interact with sugars and hemes. DOMON domains were initially thought to confer protein-protein interactions. They were subsequently found as a heme-binding motif in cellobiose dehydrogenase, an extracellular fungal oxidoreductase that degrades both lignin and cellulose, and in ethylbenzene dehydrogenase, an enzyme that aids in the anaerobic degradation of hydrocarbons. The domain interacts with sugars in the type 9 carbohydrate binding modules (CBM9), which are present in a variety of glycosyl hydrolases, and it can also be found at the N-terminus of sensor histidine kinases. |
| pfam06452 | CBM9_1 | 4.31e-07 | 943 | 1083 | 26 | 156 | Carbohydrate family 9 binding domain-like. CBM9_1 is a C-terminal domain on bacterial xylanase proteins, and it is tandemly repeated in a number of family-members. The CBM9 module binds to amorphous and crystalline cellulose and a range of soluble di- and monosaccharides as well as to cello- and xylo- oligomers of different degrees of polymerization. Comparison of the glucose and cellobiose complexes during crystallisation reveals surprising differences in binding of these two substrates by CBM9-2. Cellobiose was found to bind in a distinct orientation from glucose, while still maintaining optimal stacking and electrostatic interactions with the reducing end sugar. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| SMF73229.1 | 0.0 | 11 | 1107 | 255 | 1353 |
| QTH43804.1 | 0.0 | 2 | 1109 | 5 | 1116 |
| QNK56522.1 | 0.0 | 213 | 1107 | 593 | 1481 |
| AZS14129.1 | 1.87e-294 | 45 | 1107 | 215 | 1264 |
| AZS16815.1 | 6.08e-294 | 212 | 1107 | 301 | 1183 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5JVK_A | 5.88e-25 | 259 | 705 | 52 | 496 | Structuralinsights into a family 39 glycoside hydrolase from the gut symbiont Bacteroides cellulosilyticus WH2. [Bacteroides cellulosilyticus],5JVK_B Structural insights into a family 39 glycoside hydrolase from the gut symbiont Bacteroides cellulosilyticus WH2. [Bacteroides cellulosilyticus],5JVK_C Structural insights into a family 39 glycoside hydrolase from the gut symbiont Bacteroides cellulosilyticus WH2. [Bacteroides cellulosilyticus] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
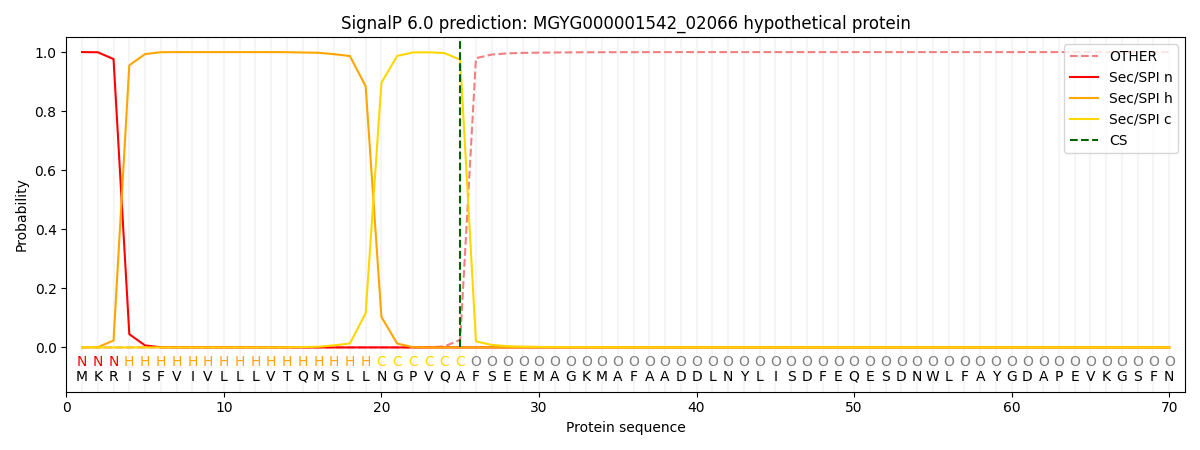
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000250 | 0.999061 | 0.000199 | 0.000172 | 0.000151 | 0.000141 |
