You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001588_00814
You are here: Home > Sequence: MGYG000001588_00814
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
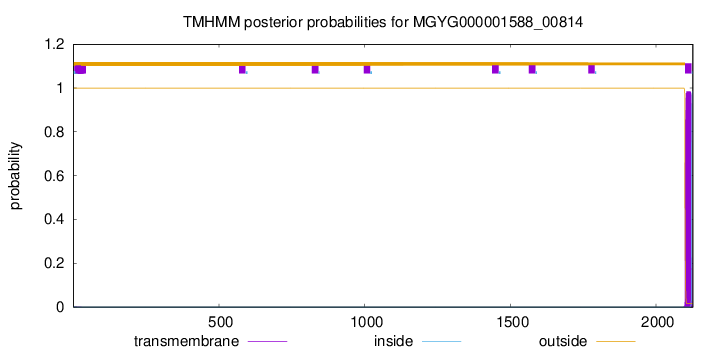
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Actinobacteriota; Coriobacteriia; Coriobacteriales; Coriobacteriaceae; Collinsella; | |||||||||||
| CAZyme ID | MGYG000001588_00814 | |||||||||||
| CAZy Family | GH31 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 15653; End: 22036 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH31 | 251 | 739 | 1.1e-47 | 0.9789227166276346 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd06596 | GH31_CPE1046 | 8.76e-134 | 261 | 710 | 1 | 334 | Clostridium CPE1046-like. CPE1046 is an uncharacterized Clostridium perfringens protein with a glycosyl hydrolase family 31 (GH31) domain. The domain architecture of CPE1046 and its orthologs includes a C-terminal fibronectin type 3 (FN3) domain and a coagulation factor 5/8 type C domain in addition to the GH31 domain. Enzymes of the GH31 family possess a wide range of different hydrolytic activities including alpha-glucosidase (glucoamylase and sucrase-isomaltase), alpha-xylosidase, 6-alpha-glucosyltransferase, 3-alpha-isomaltosyltransferase and alpha-1,4-glucan lyase. All GH31 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. |
| COG1501 | YicI | 3.90e-53 | 104 | 834 | 103 | 752 | Alpha-glucosidase, glycosyl hydrolase family GH31 [Carbohydrate transport and metabolism]. |
| pfam01055 | Glyco_hydro_31 | 7.44e-41 | 480 | 739 | 210 | 442 | Glycosyl hydrolases family 31. Glycosyl hydrolases are key enzymes of carbohydrate metabolism. Family 31 comprises of enzymes that are, or similar to, alpha- galactosidases. |
| cd08759 | Type_III_cohesin_like | 1.12e-32 | 1268 | 1437 | 1 | 167 | Cohesin domain, interaction partner of dockerin. Bacterial cohesin domains bind to a complementary protein domain named dockerin, and this interaction is required for the formation of the cellulosome, a cellulose-degrading complex. Two specific calcium-dependent interactions between cohesin and dockerin appear to be essential for cellulosome assembly, type I and type II. This subfamily represents type III cohesins and closely related domains. |
| cd06589 | GH31 | 8.23e-30 | 365 | 607 | 33 | 265 | glycosyl hydrolase family 31 (GH31). GH31 enzymes occur in prokaryotes, eukaryotes, and archaea with a wide range of hydrolytic activities, including alpha-glucosidase (glucoamylase and sucrase-isomaltase), alpha-xylosidase, 6-alpha-glucosyltransferase, 3-alpha-isomaltosyltransferase and alpha-1,4-glucan lyase. All GH31 enzymes cleave a terminal carbohydrate moiety from a substrate that varies considerably in size, depending on the enzyme, and may be either a starch or a glycoprotein. In most cases, the pyranose moiety recognized in subsite -1 of the substrate binding site is an alpha-D-glucose, though some GH31 family members show a preference for alpha-D-xylose. Several GH31 enzymes can accommodate both glucose and xylose and different levels of discrimination between the two have been observed. Most characterized GH31 enzymes are alpha-glucosidases. In mammals, GH31 members with alpha-glucosidase activity are implicated in at least three distinct biological processes. The lysosomal acid alpha-glucosidase (GAA) is essential for glycogen degradation and a deficiency or malfunction of this enzyme causes glycogen storage disease II, also known as Pompe disease. In the endoplasmic reticulum, alpha-glucosidase II catalyzes the second step in the N-linked oligosaccharide processing pathway that constitutes part of the quality control system for glycoprotein folding and maturation. The intestinal enzymes sucrase-isomaltase (SI) and maltase-glucoamylase (MGAM) play key roles in the final stage of carbohydrate digestion, making alpha-glucosidase inhibitors useful in the treatment of type 2 diabetes. GH31 alpha-glycosidases are retaining enzymes that cleave their substrates via an acid/base-catalyzed, double-displacement mechanism involving a covalent glycosyl-enzyme intermediate. Two aspartic acid residues have been identified as the catalytic nucleophile and the acid/base, respectively. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QWT17625.1 | 0.0 | 1 | 2065 | 21 | 2112 |
| BCT46261.1 | 0.0 | 38 | 2060 | 59 | 2041 |
| QNM10857.1 | 0.0 | 35 | 2061 | 57 | 2010 |
| BBK61154.1 | 0.0 | 20 | 2058 | 39 | 2067 |
| BBK23937.1 | 0.0 | 35 | 2058 | 70 | 1996 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6M76_A | 1.76e-228 | 30 | 1048 | 46 | 963 | GH31alpha-N-acetylgalactosaminidase from Enterococcus faecalis [Enterococcus faecalis ATCC 10100],6M77_A GH31 alpha-N-acetylgalactosaminidase from Enterococcus faecalis in complex with N-acetylgalactosamine [Enterococcus faecalis ATCC 10100] |
| 7F7R_A | 9.30e-228 | 30 | 1048 | 46 | 963 | ChainA, GH31 alpha-N-acetylgalactosaminidase [Enterococcus faecalis ATCC 10100] |
| 7F7Q_A | 2.53e-227 | 30 | 1048 | 46 | 963 | ChainA, GH31 alpha-N-acetylgalactosaminidase [Enterococcus faecalis ATCC 10100] |
| 5X7O_A | 1.13e-17 | 47 | 786 | 48 | 728 | Crystalstructure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase [Paenibacillus sp. 598K],5X7O_B Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase [Paenibacillus sp. 598K],5X7P_A Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase complexed with acarbose [Paenibacillus sp. 598K],5X7P_B Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase complexed with acarbose [Paenibacillus sp. 598K],5X7Q_A Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase complexed with maltohexaose [Paenibacillus sp. 598K],5X7Q_B Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase complexed with maltohexaose [Paenibacillus sp. 598K],5X7R_A Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase complexed with isomaltohexaose [Paenibacillus sp. 598K],5X7R_B Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase complexed with isomaltohexaose [Paenibacillus sp. 598K],5X7S_A Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase, terbium derivative [Paenibacillus sp. 598K],5X7S_B Crystal structure of Paenibacillus sp. 598K alpha-1,6-glucosyltransferase, terbium derivative [Paenibacillus sp. 598K] |
| 5F7C_A | 6.99e-17 | 447 | 750 | 438 | 726 | Crystalstructure of Family 31 alpha-glucosidase (BT_0339) from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482],5F7C_B Crystal structure of Family 31 alpha-glucosidase (BT_0339) from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482],5F7C_C Crystal structure of Family 31 alpha-glucosidase (BT_0339) from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482],5F7C_D Crystal structure of Family 31 alpha-glucosidase (BT_0339) from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron VPI-5482] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q9F234 | 1.37e-21 | 502 | 776 | 462 | 711 | Alpha-glucosidase 2 OS=Bacillus thermoamyloliquefaciens OX=1425 PE=3 SV=1 |
| B9F676 | 2.26e-17 | 477 | 740 | 544 | 778 | Probable glucan 1,3-alpha-glucosidase OS=Oryza sativa subsp. japonica OX=39947 GN=Os03g0216600 PE=3 SV=1 |
| Q9FN05 | 2.27e-17 | 459 | 740 | 531 | 780 | Probable glucan 1,3-alpha-glucosidase OS=Arabidopsis thaliana OX=3702 GN=PSL5 PE=1 SV=1 |
| Q8BVW0 | 1.15e-14 | 497 | 741 | 544 | 765 | Neutral alpha-glucosidase C OS=Mus musculus OX=10090 GN=Ganc PE=1 SV=2 |
| P79403 | 6.05e-14 | 502 | 742 | 596 | 813 | Neutral alpha-glucosidase AB OS=Sus scrofa OX=9823 GN=GANAB PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
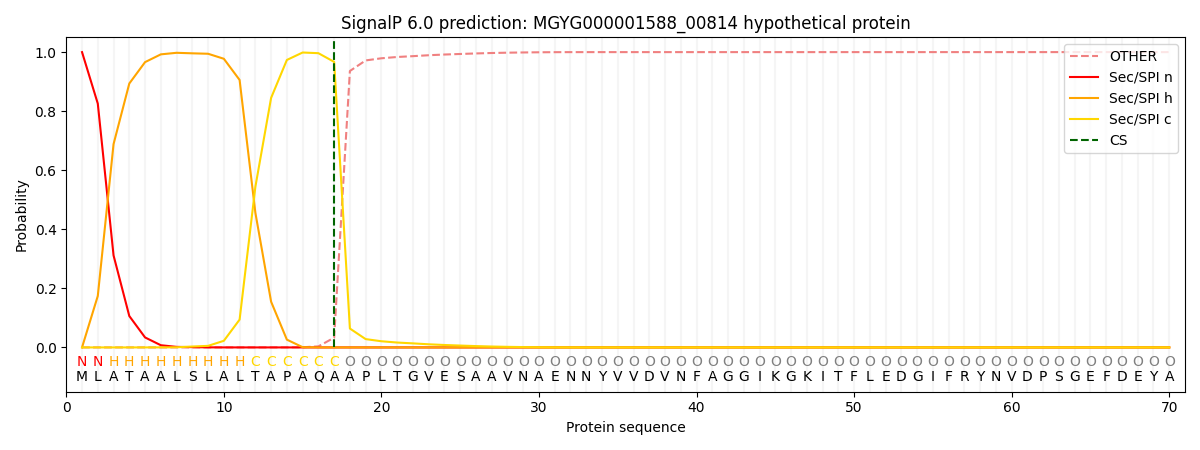
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000680 | 0.998387 | 0.000232 | 0.000267 | 0.000216 | 0.000198 |