You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001633_02011
You are here: Home > Sequence: MGYG000001633_02011
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | RC9 sp000435075 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; UBA932; RC9; RC9 sp000435075 | |||||||||||
| CAZyme ID | MGYG000001633_02011 | |||||||||||
| CAZy Family | GH29 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 11059; End: 12939 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH29 | 56 | 388 | 2.6e-72 | 0.8323699421965318 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3669 | AfuC | 4.76e-47 | 47 | 504 | 11 | 426 | Alpha-L-fucosidase [Carbohydrate transport and metabolism]. |
| pfam01120 | Alpha_L_fucos | 9.00e-32 | 62 | 388 | 30 | 326 | Alpha-L-fucosidase. |
| smart00812 | Alpha_L_fucos | 1.62e-29 | 56 | 388 | 25 | 328 | Alpha-L-fucosidase. O-Glycosyl hydrolases (EC 3.2.1.-) are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycosyl hydrolases, based on sequence similarity, has led to the definition of 85 different families. This classification is available on the CAZy (CArbohydrate-Active EnZymes) web site. Because the fold of proteins is better conserved than their sequences, some of the families can be grouped in 'clans'. Family 29 encompasses alpha-L-fucosidases, which is a lysosomal enzyme responsible for hydrolyzing the alpha-1,6-linked fucose joined to the reducing-end N-acetylglucosamine of the carbohydrate moieties of glycoproteins. Deficiency of alpha-L-fucosidase results in the lysosomal storage disease fucosidosis. |
| pfam00754 | F5_F8_type_C | 6.20e-08 | 529 | 614 | 22 | 111 | F5/8 type C domain. This domain is also known as the discoidin (DS) domain family. |
| cd00057 | FA58C | 4.08e-04 | 531 | 614 | 35 | 127 | Substituted updates: Jan 31, 2002 |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QUT50205.1 | 1.01e-255 | 7 | 618 | 6 | 698 |
| SCD22150.1 | 1.19e-251 | 3 | 618 | 1 | 727 |
| QRO25056.1 | 8.35e-249 | 12 | 618 | 19 | 735 |
| QTO26413.1 | 1.32e-244 | 24 | 618 | 26 | 722 |
| QCQ30568.1 | 7.55e-244 | 24 | 618 | 26 | 722 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 4ZRX_A | 1.07e-104 | 39 | 622 | 24 | 572 | Crystalstructure of a putative alpha-L-fucosidase (BACOVA_04357) from Bacteroides ovatus ATCC 8483 at 1.59 A resolution [Bacteroides ovatus ATCC 8483] |
| 3UES_A | 2.74e-97 | 34 | 507 | 6 | 475 | Crystalstructure of alpha-1,3/4-fucosidase from Bifidobacterium longum subsp. infantis complexed with deoxyfuconojirimycin [Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 = DSM 20088],3UES_B Crystal structure of alpha-1,3/4-fucosidase from Bifidobacterium longum subsp. infantis complexed with deoxyfuconojirimycin [Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 = DSM 20088] |
| 3MO4_A | 3.17e-96 | 34 | 507 | 8 | 477 | Thecrystal structure of an alpha-(1-3,4)-fucosidase from Bifidobacterium longum subsp. infantis ATCC 15697 [Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 = DSM 20088],3MO4_B The crystal structure of an alpha-(1-3,4)-fucosidase from Bifidobacterium longum subsp. infantis ATCC 15697 [Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 = DSM 20088] |
| 3UET_A | 3.25e-95 | 34 | 507 | 6 | 475 | Crystalstructure of alpha-1,3/4-fucosidase from Bifidobacterium longum subsp. infantis D172A/E217A mutant complexed with lacto-N-fucopentaose II [Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 = DSM 20088],3UET_B Crystal structure of alpha-1,3/4-fucosidase from Bifidobacterium longum subsp. infantis D172A/E217A mutant complexed with lacto-N-fucopentaose II [Bifidobacterium longum subsp. infantis ATCC 15697 = JCM 1222 = DSM 20088] |
| 3EYP_A | 6.68e-82 | 45 | 502 | 8 | 448 | Crystalstructure of putative alpha-L-fucosidase from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron],3EYP_B Crystal structure of putative alpha-L-fucosidase from Bacteroides thetaiotaomicron [Bacteroides thetaiotaomicron],4OUE_A Crystal structure of an a-L-Fucosidase GH29 from Bacteroides thetaiotaomicron (BT2192) in complex with IPTG [Bacteroides thetaiotaomicron VPI-5482],4OUE_B Crystal structure of an a-L-Fucosidase GH29 from Bacteroides thetaiotaomicron (BT2192) in complex with IPTG [Bacteroides thetaiotaomicron VPI-5482],4OZO_A Crystal structure of an a-L-fucosidase GH29 from Bacteroides thetaiotaomicron (BT2192) in complex with oNPTG [Bacteroides thetaiotaomicron VPI-5482],4OZO_B Crystal structure of an a-L-fucosidase GH29 from Bacteroides thetaiotaomicron (BT2192) in complex with oNPTG [Bacteroides thetaiotaomicron VPI-5482] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q8GW72 | 4.72e-76 | 46 | 508 | 37 | 477 | Alpha-L-fucosidase 1 OS=Arabidopsis thaliana OX=3702 GN=FUC1 PE=1 SV=2 |
| Q7XUR3 | 4.28e-68 | 46 | 544 | 39 | 503 | Putative alpha-L-fucosidase 1 OS=Oryza sativa subsp. japonica OX=39947 GN=Os04g0560400 PE=3 SV=2 |
| P49713 | 7.43e-12 | 82 | 393 | 89 | 345 | Putative alpha-L-fucosidase OS=Caenorhabditis elegans OX=6239 GN=W03G11.3 PE=3 SV=2 |
| P10901 | 9.66e-08 | 73 | 387 | 85 | 350 | Alpha-L-fucosidase OS=Dictyostelium discoideum OX=44689 GN=alfA PE=3 SV=1 |
| Q99KR8 | 2.74e-06 | 70 | 191 | 83 | 209 | Plasma alpha-L-fucosidase OS=Mus musculus OX=10090 GN=Fuca2 PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as LIPO
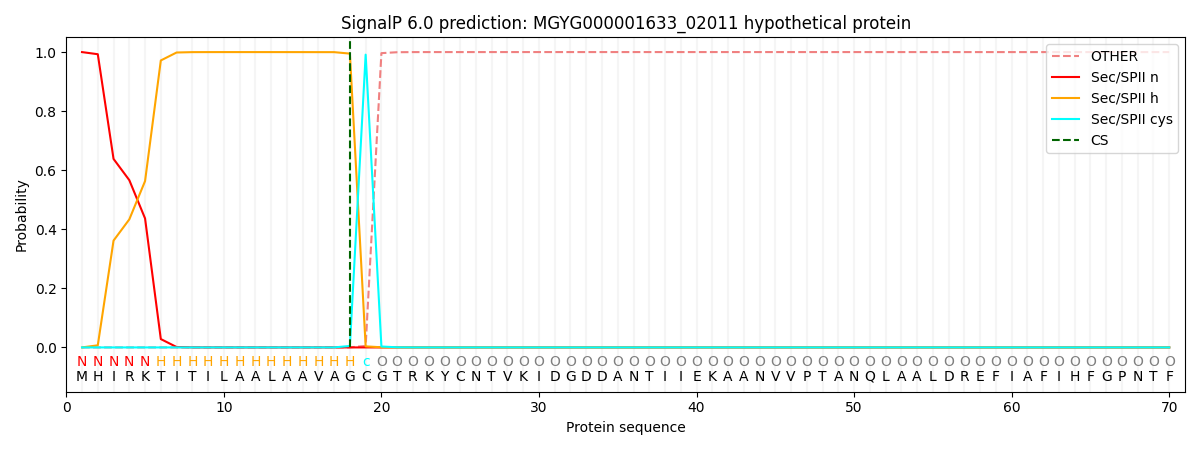
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000000 | 0.000001 | 1.000060 | 0.000000 | 0.000000 | 0.000000 |
