You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001660_00348
You are here: Home > Sequence: MGYG000001660_00348
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | HGM11788 sp900760465 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Lachnospirales; CAG-274; HGM11788; HGM11788 sp900760465 | |||||||||||
| CAZyme ID | MGYG000001660_00348 | |||||||||||
| CAZy Family | PL1 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 19019; End: 23227 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| PL1 | 240 | 434 | 1.5e-32 | 0.8762376237623762 |
| CBM77 | 787 | 886 | 2.5e-16 | 0.9514563106796117 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| COG3866 | PelB | 1.05e-24 | 251 | 524 | 98 | 340 | Pectate lyase [Carbohydrate transport and metabolism]. |
| smart00656 | Amb_all | 1.74e-15 | 253 | 436 | 15 | 190 | Amb_all domain. |
| pfam00404 | Dockerin_1 | 2.17e-11 | 1339 | 1393 | 1 | 56 | Dockerin type I repeat. The dockerin repeat is the binding partner of the cohesin domain pfam00963. The cohesin-dockerin interaction is the crucial interaction for complex formation in the cellulosome. The dockerin repeats, each bearing homology to the EF-hand calcium-binding loop bind calcium. |
| cd14256 | Dockerin_I | 5.31e-11 | 1338 | 1393 | 1 | 57 | Type I dockerin repeat domain. Bacterial cohesin domains bind to a complementary protein domain named dockerin, and this interaction is required for the formation of the cellulosome, a cellulose-degrading complex. The cellulosome consists of scaffoldin, a noncatalytic scaffolding polypeptide, that comprises repeating cohesion modules and a single carbohydrate-binding module (CBM). Specific calcium-dependent interactions between cohesins and dockerins appear to be essential for cellulosome assembly. This subfamily represents type I dockerins, which are responsible for anchoring a variety of enzymatic domains to the complex. |
| pfam00544 | Pec_lyase_C | 1.18e-08 | 290 | 432 | 64 | 211 | Pectate lyase. This enzyme forms a right handed beta helix structure. Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| BBF42492.1 | 1.76e-131 | 1 | 619 | 1 | 612 |
| ADU23076.1 | 6.23e-97 | 37 | 528 | 763 | 1239 |
| ACR71161.1 | 6.60e-96 | 37 | 891 | 47 | 890 |
| CDR31241.1 | 1.84e-86 | 35 | 531 | 333 | 812 |
| QEH68115.1 | 2.70e-69 | 1 | 527 | 2 | 478 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 3VMV_A | 3.55e-15 | 254 | 437 | 79 | 251 | Crystalstructure of pectate lyase Bsp165PelA from Bacillus sp. N165 [Bacillus sp. N16-5],3VMW_A Crystal structure of pectate lyase Bsp165PelA from Bacillus sp. N165 in complex with trigalacturonate [Bacillus sp. N16-5] |
| 1AIR_A | 1.19e-12 | 211 | 433 | 50 | 258 | ChainA, PECTATE LYASE C [Dickeya chrysanthemi],1O88_A Chain A, Pectate Lyase C [Dickeya chrysanthemi],1O8D_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8E_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8F_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8G_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8H_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8I_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8J_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8K_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8L_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1O8M_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi],1PLU_A Chain A, Protein (pectate Lyase C) [Dickeya chrysanthemi],2PEC_A Chain A, PECTATE LYASE C [Dickeya chrysanthemi] |
| 5AMV_A | 1.67e-12 | 229 | 416 | 102 | 302 | Structuralinsights into the loss of catalytic competence in pectate lyase at low pH [Bacillus subtilis],5X2I_A Polygalacturonate Lyase by Fusing with a Self-assembling Amphipathic Peptide [Bacillus subtilis subsp. subtilis str. 168] |
| 1BN8_A | 1.89e-12 | 229 | 416 | 123 | 323 | BacillusSubtilis Pectate Lyase [Bacillus subtilis] |
| 2EWE_A | 2.84e-12 | 211 | 433 | 50 | 258 | ChainA, Pectate lyase C [Dickeya chrysanthemi] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P04959 | 4.39e-14 | 211 | 442 | 71 | 289 | Pectate lyase B OS=Dickeya chrysanthemi OX=556 GN=pelB PE=3 SV=1 |
| B1B6T1 | 5.58e-14 | 254 | 436 | 108 | 276 | Pectate trisaccharide-lyase OS=Bacillus sp. OX=1409 GN=pel PE=1 SV=1 |
| Q65DC2 | 5.58e-14 | 254 | 436 | 108 | 276 | Pectate trisaccharide-lyase OS=Bacillus licheniformis (strain ATCC 14580 / DSM 13 / JCM 2505 / CCUG 7422 / NBRC 12200 / NCIMB 9375 / NCTC 10341 / NRRL NRS-1264 / Gibson 46) OX=279010 GN=BLi04129 PE=3 SV=1 |
| Q8GCB2 | 5.58e-14 | 254 | 436 | 108 | 276 | Pectate trisaccharide-lyase OS=Bacillus licheniformis OX=1402 GN=pelA PE=1 SV=1 |
| P0C1C1 | 1.38e-13 | 211 | 527 | 71 | 363 | Pectate lyase 2 OS=Pectobacterium carotovorum OX=554 GN=pel2 PE=3 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
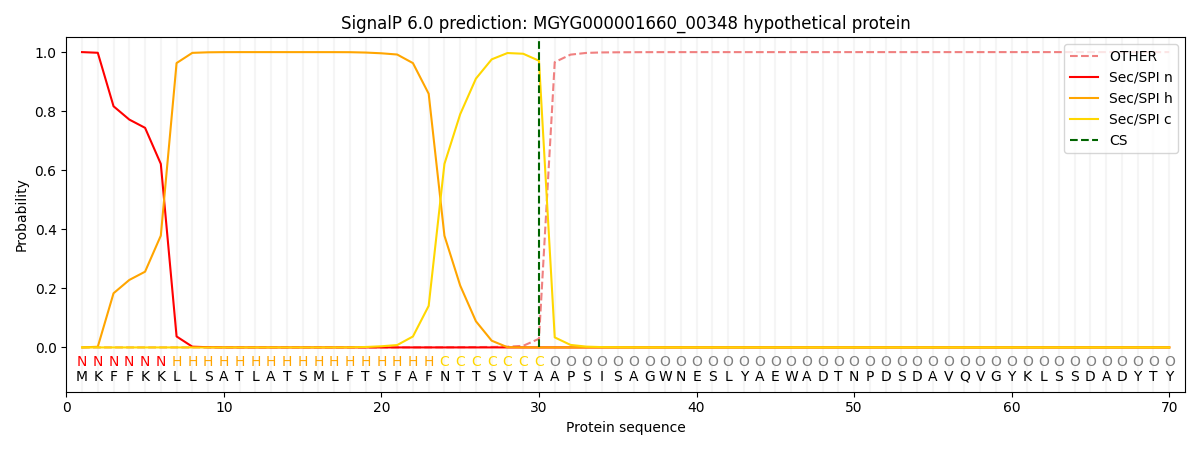
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000287 | 0.998927 | 0.000209 | 0.000207 | 0.000168 | 0.000151 |
