You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001672_00241
You are here: Home > Sequence: MGYG000001672_00241
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
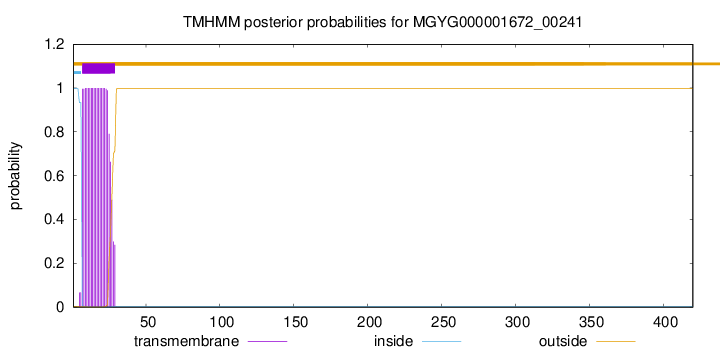
TMHMM annotations
Basic Information help
| Species | UMGS995 sp900547465 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; RF39; UBA660; UMGS995; UMGS995 sp900547465 | |||||||||||
| CAZyme ID | MGYG000001672_00241 | |||||||||||
| CAZy Family | CE4 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 32030; End: 33292 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| CE4 | 211 | 317 | 6.6e-19 | 0.8307692307692308 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd10944 | CE4_SmPgdA_like | 1.25e-66 | 217 | 404 | 2 | 187 | Catalytic NodB homology domain of Streptococcus mutans polysaccharide deacetylase PgdA, Bacillus subtilis YheN, and similar proteins. This family is represented by a putative polysaccharide deacetylase PgdA from the oral pathogen Streptococcus mutans (SmPgdA) and Bacillus subtilis YheN (BsYheN), which are members of the carbohydrate esterase 4 (CE4) superfamily. SmPgdA is an extracellular metal-dependent polysaccharide deacetylase with a typical CE4 fold, with metal bound to a His-His-Asp triad. It possesses de-N-acetylase activity toward a hexamer of chitooligosaccharide N-acetylglucosamine, but not shorter chitooligosaccharides or a synthetic peptidoglycan tetrasaccharide. SmPgdA plays a role in tuning cell surface properties and in interactions with (salivary) agglutinin, an essential component of the innate immune system, most likely through deacetylation of an as-yet-unidentified polysaccharide. SmPgdA shows significant homology to the catalytic domains of peptidoglycan deacetylases from Streptococcus pneumoniae (SpPgdA) and Listeria monocytogenes (LmPgdA), both of which are involved in the bacterial defense mechanism against human mucosal lysozyme. The Bacillus subtilis genome contains six polysaccharide deacetylase gene homologs: pdaA, pdaB (previously known as ybaN), yheN, yjeA, yxkH and ylxY. The biological function of BsYheN is still unknown. This family also includes many uncharacterized polysaccharide deacetylases mainly found in bacteria. |
| cd10917 | CE4_NodB_like_6s_7s | 8.99e-40 | 216 | 398 | 1 | 171 | Catalytic NodB homology domain of rhizobial NodB-like proteins. This family belongs to the large and functionally diverse carbohydrate esterase 4 (CE4) superfamily, whose members show strong sequence similarity with some variability due to their distinct carbohydrate substrates. It includes many rhizobial NodB chitooligosaccharide N-deacetylase (EC 3.5.1.-)-like proteins, mainly from bacteria and eukaryotes, such as chitin deacetylases (EC 3.5.1.41), bacterial peptidoglycan N-acetylglucosamine deacetylases (EC 3.5.1.-), and acetylxylan esterases (EC 3.1.1.72), which catalyze the N- or O-deacetylation of substrates such as acetylated chitin, peptidoglycan, and acetylated xylan. All members of this family contain a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold with 6- or 7 strands. Their catalytic activity is dependent on the presence of a divalent cation, preferably cobalt or zinc, and they employ a conserved His-His-Asp zinc-binding triad closely associated with the conserved catalytic base (aspartic acid) and acid (histidine) to carry out acid/base catalysis. Several family members show diversity both in metal ion specificities and in the residues that coordinate the metal. |
| COG0726 | CDA1 | 4.05e-31 | 213 | 404 | 62 | 250 | Peptidoglycan/xylan/chitin deacetylase, PgdA/CDA1 family [Carbohydrate transport and metabolism, Cell wall/membrane/envelope biogenesis]. |
| cd10954 | CE4_CtAXE_like | 1.67e-26 | 217 | 404 | 2 | 174 | Catalytic NodB homology domain of Clostridium thermocellum acetylxylan esterase and its bacterial homologs. This family is represented by Clostridium thermocellum acetylxylan esterase (CtAXE, EC 3.1.1.72), a member of the carbohydrate esterase 4 (CE4) superfamily. CtAXE deacetylates O-acetylated xylan, a key component of plant cell walls. It shows no detectable activity on generic esterase substrates including para-nitrophenyl acetate. It is specific for sugar-based substrates and will precipitate acetylxylan, as a consequence of deacetylation. CtAXE is a monomeric protein containing a catalytic NodB homology domain with the same overall topology and a deformed (beta/alpha)8 barrel fold as other CE4 esterases. However, due to differences in the topography of the substrate-binding groove, the chemistry of the active center, and metal ion coordination, CtAXE has different metal ion preference and lacks activity on N-acetyl substrates. It is significantly activated by Co2+. Moreover, CtAXE displays distinctly different ligand coordination to the metal ion, utilizing an aspartate, a histidine, and four water molecules, as opposed to the conserved His-His-Asp zinc-binding triad of other CE4 esterases. |
| cd10962 | CE4_GT2-like | 2.50e-25 | 218 | 409 | 3 | 193 | Catalytic NodB homology domain of uncharacterized bacterial glycosyl transferase, group 2-like family proteins. This family includes many uncharacterized bacterial proteins containing an N-terminal GH18 (glycosyl hydrolase, family 18) domain, a middle NodB-like homology domain, and a C-terminal GT2-like (glycosyl transferase group 2) domain. Although their biological function is unknown, members in this family contain a middle NodB homology domain that is similar to the catalytic domain of Streptococcus pneumoniae polysaccharide deacetylase PgdA (SpPgdA), an extracellular metal-dependent polysaccharide deacetylase with de-N-acetylase activity toward a hexamer of chitooligosaccharide N-acetylglucosamine, but not shorter chitooligosaccharides or a synthetic peptidoglycan tetrasaccharide. Like SpPgdA, this family is a member of the carbohydrate esterase 4 (CE4) superfamily. The presence of three domains suggests that members of this family may be multifunctional. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| CBK81881.1 | 5.16e-76 | 41 | 420 | 89 | 477 |
| CBK92741.1 | 1.95e-71 | 74 | 420 | 134 | 489 |
| ACR76120.1 | 2.31e-71 | 74 | 420 | 141 | 496 |
| CBK91697.1 | 2.73e-71 | 110 | 420 | 168 | 489 |
| ACV23495.1 | 1.15e-69 | 110 | 420 | 186 | 503 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5JMU_A | 2.13e-50 | 213 | 420 | 17 | 226 | ChainA, Peptidoglycan N-acetylglucosamine deacetylase [[Eubacterium] rectale ATCC 33656] |
| 5NC9_A | 3.36e-24 | 196 | 405 | 22 | 229 | Crystalstructure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (2S)-2,6-diamino-N-hydroxyhexanamide [Bacillus cereus ATCC 14579],5NC9_B Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (2S)-2,6-diamino-N-hydroxyhexanamide [Bacillus cereus ATCC 14579],5NC9_C Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (2S)-2,6-diamino-N-hydroxyhexanamide [Bacillus cereus ATCC 14579],5NC9_D Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (2S)-2,6-diamino-N-hydroxyhexanamide [Bacillus cereus ATCC 14579],5NCD_A Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (2S)-2-amino-5-(diaminomethylideneamino)-N-hydroxypentanamide [Bacillus cereus],5NCD_B Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (2S)-2-amino-5-(diaminomethylideneamino)-N-hydroxypentanamide [Bacillus cereus],5NCD_C Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (2S)-2-amino-5-(diaminomethylideneamino)-N-hydroxypentanamide [Bacillus cereus],5NCD_D Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (2S)-2-amino-5-(diaminomethylideneamino)-N-hydroxypentanamide [Bacillus cereus],5NEK_A Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with acetazolamide [Bacillus cereus],5NEK_B Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with acetazolamide [Bacillus cereus],5NEK_C Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with acetazolamide [Bacillus cereus],5NEK_D Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with acetazolamide [Bacillus cereus],5NEL_A Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with ThiametG [Bacillus cereus],5NEL_B Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with ThiametG [Bacillus cereus],5NEL_C Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with ThiametG [Bacillus cereus],5NEL_D Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with ThiametG [Bacillus cereus] |
| 5NC6_B | 5.68e-24 | 196 | 405 | 48 | 255 | Crystalstructure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (E)-N-hydroxy-3-(naphthalen-1-yl)prop-2-enamide [Bacillus cereus],5NC6_C Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (E)-N-hydroxy-3-(naphthalen-1-yl)prop-2-enamide [Bacillus cereus],5NC6_D Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (E)-N-hydroxy-3-(naphthalen-1-yl)prop-2-enamide [Bacillus cereus] |
| 5NC6_A | 1.24e-23 | 217 | 405 | 2 | 187 | Crystalstructure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with (E)-N-hydroxy-3-(naphthalen-1-yl)prop-2-enamide [Bacillus cereus] |
| 5N1J_A | 1.27e-23 | 217 | 405 | 3 | 188 | Crystalstructure of the polysaccharide deacetylase Bc1974 from Bacillus cereus [Bacillus cereus],5N1J_B Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus [Bacillus cereus],5N1J_C Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus [Bacillus cereus],5N1J_D Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus [Bacillus cereus],5N1P_A Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with N-hydroxynaphthalene-1-carboxamide [Bacillus cereus],5N1P_B Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with N-hydroxynaphthalene-1-carboxamide [Bacillus cereus],5N1P_C Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with N-hydroxynaphthalene-1-carboxamide [Bacillus cereus],5N1P_D Crystal structure of the polysaccharide deacetylase Bc1974 from Bacillus cereus in complex with N-hydroxynaphthalene-1-carboxamide [Bacillus cereus] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| Q81EJ6 | 3.11e-23 | 196 | 405 | 48 | 255 | Peptidoglycan-N-acetylglucosamine deacetylase BC_1974 OS=Bacillus cereus (strain ATCC 14579 / DSM 31 / CCUG 7414 / JCM 2152 / NBRC 15305 / NCIMB 9373 / NCTC 2599 / NRRL B-3711) OX=226900 GN=BC_1974 PE=1 SV=1 |
| O07596 | 1.19e-21 | 218 | 404 | 87 | 270 | Putative polysaccharide deacetylase YheN OS=Bacillus subtilis (strain 168) OX=224308 GN=yheN PE=3 SV=1 |
| Q04729 | 3.05e-16 | 213 | 418 | 64 | 258 | Uncharacterized 30.6 kDa protein in fumA 3'region OS=Geobacillus stearothermophilus OX=1422 PE=3 SV=1 |
| O34798 | 5.45e-14 | 144 | 417 | 209 | 464 | Peptidoglycan-N-acetylmuramic acid deacetylase PdaC OS=Bacillus subtilis (strain 168) OX=224308 GN=pdaC PE=1 SV=1 |
| A0A3Q0NBH7 | 4.08e-13 | 168 | 404 | 218 | 439 | Peptidoglycan-N-acetylglucosamine deacetylase PgdA OS=Listeria monocytogenes serotype 1/2a (strain EGD / Mackaness) OX=1334565 GN=pgdA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as OTHER
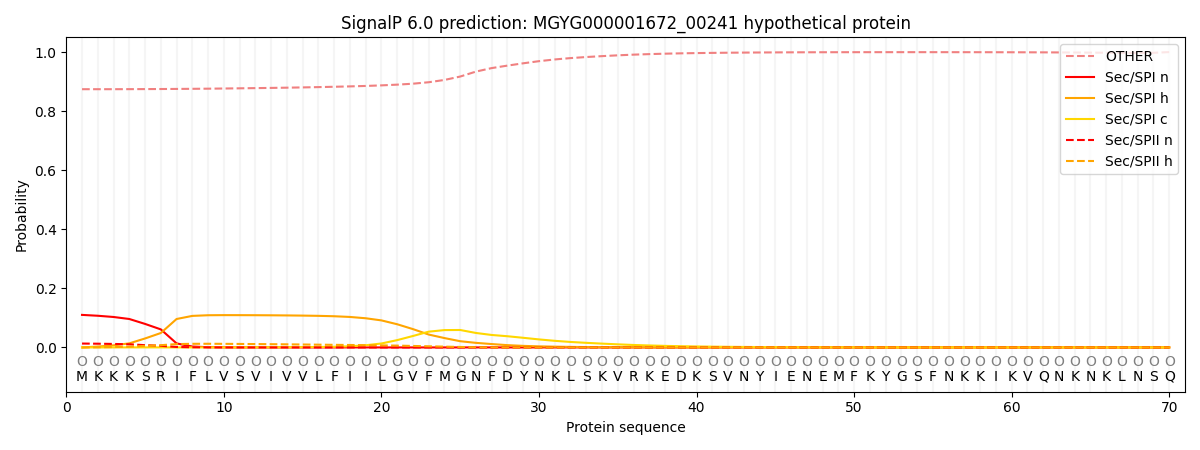
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.880446 | 0.102125 | 0.013757 | 0.000419 | 0.000263 | 0.003000 |