You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001694_00342
You are here: Home > Sequence: MGYG000001694_00342
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | Enterococcus faecalis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes; Bacilli; Lactobacillales; Enterococcaceae; Enterococcus; Enterococcus faecalis | |||||||||||
| CAZyme ID | MGYG000001694_00342 | |||||||||||
| CAZy Family | GH23 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 344241; End: 344840 Strand: + | |||||||||||
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| smart00257 | LysM | 3.21e-07 | 31 | 76 | 2 | 44 | Lysin motif. |
| cd00118 | LysM | 4.03e-07 | 31 | 76 | 3 | 45 | Lysin Motif is a small domain involved in binding peptidoglycan. LysM, a small globular domain with approximately 40 amino acids, is a widespread protein module involved in binding peptidoglycan in bacteria and chitin in eukaryotes. The domain was originally identified in enzymes that degrade bacterial cell walls, but proteins involved in many other biological functions also contain this domain. It has been reported that the LysM domain functions as a signal for specific plant-bacteria recognition in bacterial pathogenesis. Many of these enzymes are modular and are composed of catalytic units linked to one or several repeats of LysM domains. LysM domains are found in bacteria and eukaryotes. |
| pfam01476 | LysM | 1.87e-06 | 31 | 76 | 1 | 42 | LysM domain. The LysM (lysin motif) domain is about 40 residues long. It is found in a variety of enzymes involved in bacterial cell wall degradation. This domain may have a general peptidoglycan binding function. The structure of this domain is known. |
| PRK11198 | PRK11198 | 1.17e-05 | 31 | 76 | 98 | 145 | LysM domain/BON superfamily protein; Provisional |
| COG1652 | XkdP | 0.003 | 14 | 76 | 196 | 261 | Nucleoid-associated protein YgaU, contains BON and LysM domains [Function unknown]. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QFY91597.1 | 3.92e-118 | 1 | 199 | 1 | 199 |
| QDJ05304.1 | 3.92e-118 | 1 | 199 | 1 | 199 |
| QSO29449.1 | 3.92e-118 | 1 | 199 | 1 | 199 |
| AEA93015.1 | 3.92e-118 | 1 | 199 | 1 | 199 |
| QVW73775.1 | 3.92e-118 | 1 | 199 | 1 | 199 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5FIM_A | 1.15e-06 | 23 | 81 | 91 | 151 | Thestructure of Kbp.K from E. coli [Escherichia coli],7PVC_A Chain A, Potassium binding protein Kbp [Escherichia coli K-12] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
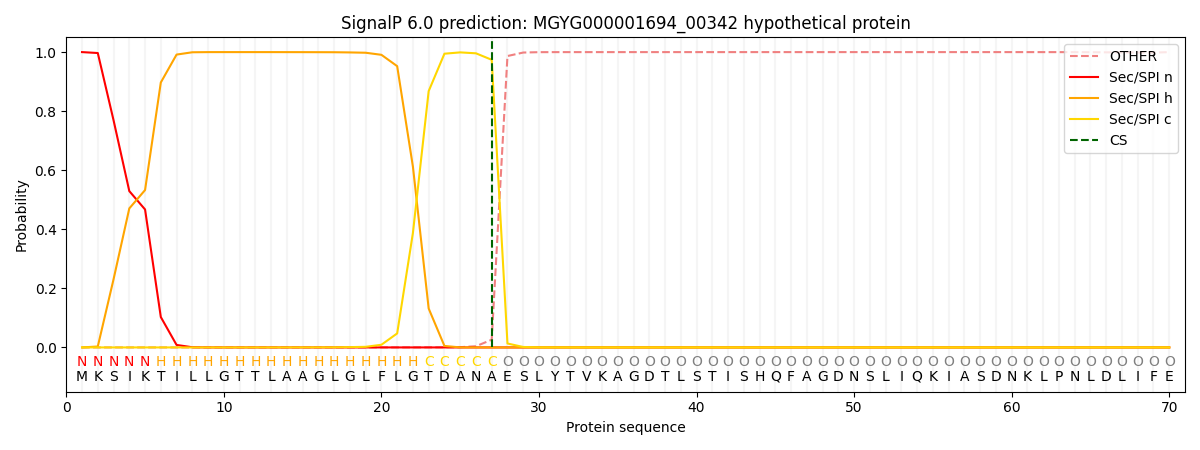
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.001359 | 0.997308 | 0.000352 | 0.000356 | 0.000308 | 0.000302 |
