You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001732_01028
You are here: Home > Sequence: MGYG000001732_01028
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
TMHMM annotations
Basic Information help
| Species | HGM11530 sp900751685 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Firmicutes_A; Clostridia; Monoglobales_A; UBA1381; HGM11530; HGM11530 sp900751685 | |||||||||||
| CAZyme ID | MGYG000001732_01028 | |||||||||||
| CAZy Family | GH43 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 3335; End: 8668 Strand: + | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH51 | 1213 | 1759 | 1.9e-118 | 0.8174603174603174 |
| GH43 | 50 | 350 | 8e-62 | 0.996309963099631 |
| CBM57 | 653 | 783 | 6.1e-17 | 0.9727891156462585 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| cd09001 | GH43_FsAxh1-like | 1.36e-77 | 49 | 353 | 2 | 270 | Glycosyl hydrolase family 43 such as Fibrobacter succinogenes subsp. succinogenes S85 arabinoxylan alpha-L-arabinofuranosidase. This glycosyl hydrolase family 43 (GH43) includes mostly enzymes that have been annotated as having beta-1,4-xylosidase (beta-D-xylosidase; xylan 1,4-beta-xylosidase; EC 3.2.1.37) activity. They are part of an array of hemicellulases that are involved in the final breakdown of plant cell-wall whereby they degrade xylan. They hydrolyze beta-1,4 glycosidic bonds between two xylose units in short xylooligosaccharides. These are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. This subfamily includes the characterized Clostridium stercorarium F-9 beta-xylosidase Xyl43B. It also includes Humicola insolens AXHd3 (HiAXHd3), a GH43 arabinofuranosidase (EC 3.2.1.55) that hydrolyzes O3-linked arabinose of doubly substituted xylans, a feature of the polysaccharide that is recalcitrant to degradation. It possesses an additional C-terminal beta-sandwich domain such that the interface between the domains comprises a xylan binding cleft that houses the active site pocket. The HiAXHd3 active site is tuned to hydrolyze arabinofuranosyl or xylosyl linkages, and the topology of the distal regions of the substrate binding surface confers specificity. It also includes Fibrobacter succinogenes subsp. succinogenes S85 arabinoxylan alpha-L-arabinofuranosidase (Axh1;Fisuc_1769;FSU_2269), Paenibacillus sp. E18 alpha-L-arabinofuranosidase (Abf43A), Bifidobacterium adolescentis ATCC 15703 double substituted xylan alpha-1,3-L-specific arabinofuranosidase d3 (AXHd3;AXH-d3;BaAXH-d3;BAD_0301;E-AFAM2), and Chrysosporium lucknowense C1 arabinoxylan hydrolase / double substituted xylan alpha-1,3-L-arabinofuranosidase (Abn7;AXHd). A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
| COG3534 | AbfA | 3.92e-57 | 1221 | 1677 | 33 | 501 | Alpha-L-arabinofuranosidase [Carbohydrate transport and metabolism]. |
| pfam06964 | Alpha-L-AF_C | 3.30e-45 | 1482 | 1668 | 1 | 192 | Alpha-L-arabinofuranosidase C-terminal domain. This family represents the C-terminus (approximately 200 residues) of bacterial and eukaryotic alpha-L-arabinofuranosidase (EC:3.2.1.55). This catalyzes the hydrolysis of nonreducing terminal alpha-L-arabinofuranosidic linkages in L-arabinose-containing polysaccharides. |
| COG3507 | XynB2 | 1.27e-44 | 43 | 570 | 15 | 547 | Beta-xylosidase [Carbohydrate transport and metabolism]. |
| cd08989 | GH43_XYL-like | 1.86e-44 | 51 | 344 | 1 | 271 | Glycosyl hydrolase family 43, beta-D-xylosidases and arabinofuranosidases. This glycosyl hydrolase family 43 (GH43) subgroup includes mostly enzymes that have been annotated as having beta-1,4-xylosidase (beta-D-xylosidase;xylan 1,4-beta-xylosidase; EC 3.2.1.37) activity, including Selenomonas ruminantium beta-D-xylosidase SXA. These are part of an array of hemicellulases that are involved in the final breakdown of plant cell-wall whereby they degrade xylan. They hydrolyze beta-1,4 glycosidic bonds between two xylose units in short xylooligosaccharides. It also includes various GH43 family GH43 arabinofuranosidases (EC 3.2.1.55) including Humicola insolens alpha-L-arabinofuranosidase AXHd3, Bacteroides ovatus alpha-L-arabinofuranosidase (BoGH43, XynB), and the bifunctional Phanerochaete chrysosporium xylosidase/arabinofuranosidase (Xyl;PcXyl). GH43 are inverting enzymes (i.e. they invert the stereochemistry of the anomeric carbon atom of the substrate) that have an aspartate as the catalytic general base, a glutamate as the catalytic general acid and another aspartate that is responsible for pKa modulation and orienting the catalytic acid. Many GH43 enzymes display both alpha-L-arabinofuranosidase and beta-D-xylosidase activity using aryl-glycosides as substrates. A common structural feature of GH43 enzymes is a 5-bladed beta-propeller domain that contains the catalytic acid and catalytic base. A long V-shaped groove, partially enclosed at one end, forms a single extended substrate-binding surface across the face of the propeller. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| QYR23081.1 | 1.96e-192 | 39 | 570 | 19 | 550 |
| AMP97575.1 | 1.75e-187 | 1029 | 1676 | 22 | 658 |
| AHW58581.1 | 4.92e-183 | 1032 | 1676 | 22 | 644 |
| QIA07679.1 | 1.87e-182 | 1032 | 1678 | 22 | 646 |
| QDH78970.1 | 1.61e-179 | 1039 | 1678 | 35 | 659 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 6ZPS_AAA | 6.44e-81 | 1048 | 1677 | 17 | 627 | ChainAAA, MgGH51 [Meripilus giganteus],6ZPV_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZPW_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZPX_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZPY_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZPZ_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZQ0_AAA Chain AAA, MgGH51 [Meripilus giganteus],6ZQ1_AAA Chain AAA, MgGH51 [Meripilus giganteus] |
| 6MLY_A | 3.12e-41 | 51 | 575 | 23 | 516 | ChainA, Bifunctional GH43-CE protein [Bacteroides eggerthii],6MLY_B Chain B, Bifunctional GH43-CE protein [Bacteroides eggerthii],6MLY_C Chain C, Bifunctional GH43-CE protein [Bacteroides eggerthii],6MLY_D Chain D, Bifunctional GH43-CE protein [Bacteroides eggerthii] |
| 6MS3_A | 1.36e-32 | 45 | 465 | 26 | 419 | Crystalstructure of the GH43 protein BlXynB mutant (K247S) from Bacillus licheniformis [Bacillus licheniformis DSM 13 = ATCC 14580],6MS3_B Crystal structure of the GH43 protein BlXynB mutant (K247S) from Bacillus licheniformis [Bacillus licheniformis DSM 13 = ATCC 14580] |
| 6MS2_A | 1.82e-32 | 45 | 465 | 26 | 419 | Crystalstructure of the GH43 BlXynB protein from Bacillus licheniformis [Bacillus licheniformis DSM 13 = ATCC 14580] |
| 1YRZ_A | 5.31e-31 | 51 | 465 | 7 | 403 | ChainA, xylan beta-1,4-xylosidase [Halalkalibacterium halodurans C-125],1YRZ_B Chain B, xylan beta-1,4-xylosidase [Halalkalibacterium halodurans C-125] |
Swiss-Prot Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| P82593 | 4.62e-128 | 1019 | 1592 | 23 | 577 | Extracellular exo-alpha-L-arabinofuranosidase OS=Streptomyces chartreusis OX=1969 PE=1 SV=1 |
| Q9SG80 | 9.78e-89 | 1041 | 1680 | 52 | 661 | Alpha-L-arabinofuranosidase 1 OS=Arabidopsis thaliana OX=3702 GN=ASD1 PE=1 SV=1 |
| Q8VZR2 | 4.05e-88 | 1044 | 1697 | 54 | 672 | Alpha-L-arabinofuranosidase 2 OS=Arabidopsis thaliana OX=3702 GN=ASD2 PE=2 SV=1 |
| Q8NK90 | 4.64e-70 | 1048 | 1664 | 42 | 617 | Alpha-L-arabinofuranosidase A OS=Aspergillus kawachii (strain NBRC 4308) OX=1033177 GN=abfA PE=1 SV=2 |
| U6A629 | 4.95e-70 | 1033 | 1545 | 18 | 505 | Alpha-L-arabinofuranosidase A OS=Penicillium canescens OX=5083 GN=abfA PE=1 SV=1 |
SignalP and Lipop Annotations help
This protein is predicted as SP
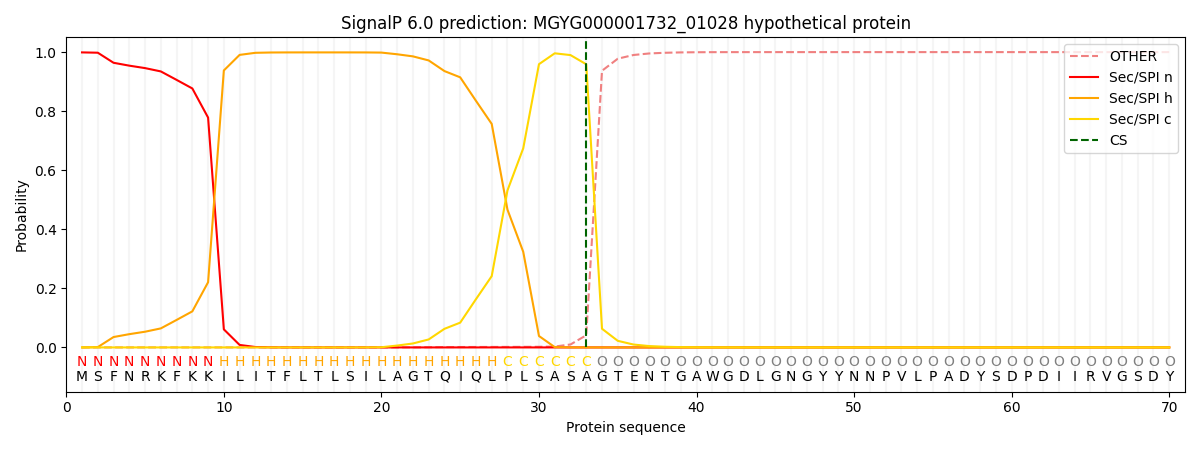
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.000623 | 0.997166 | 0.001552 | 0.000250 | 0.000199 | 0.000180 |
