You are browsing environment: HUMAN GUT
CAZyme Information: MGYG000001735_02657
You are here: Home > Sequence: MGYG000001735_02657
Basic Information |
Genomic context |
Full Sequence |
Enzyme annotations |
CAZy signature domains |
CDD domains |
CAZyme hits |
PDB hits |
Swiss-Prot hits |
SignalP and Lipop annotations |
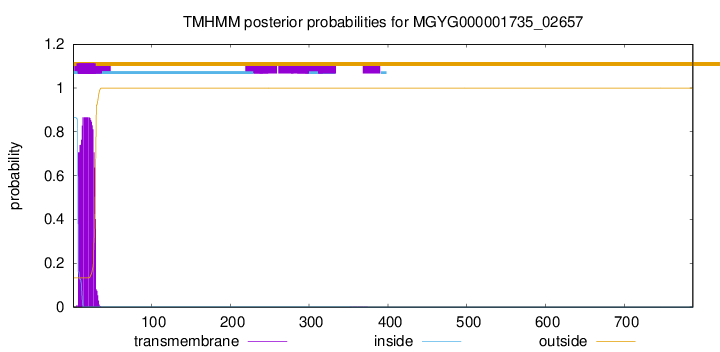
TMHMM annotations
Basic Information help
| Species | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineage | Bacteria; Bacteroidota; Bacteroidia; Bacteroidales; Tannerellaceae; Parabacteroides; | |||||||||||
| CAZyme ID | MGYG000001735_02657 | |||||||||||
| CAZy Family | GH141 | |||||||||||
| CAZyme Description | hypothetical protein | |||||||||||
| CAZyme Property |
|
|||||||||||
| Genome Property |
|
|||||||||||
| Gene Location | Start: 32963; End: 35326 Strand: - | |||||||||||
CAZyme Signature Domains help
| Family | Start | End | Evalue | family coverage |
|---|---|---|---|---|
| GH141 | 30 | 543 | 3.4e-55 | 0.9867172675521821 |
CDD Domains download full data without filtering help
| Cdd ID | Domain | E-Value | qStart | qEnd | sStart | sEnd | Domain Description |
|---|---|---|---|---|---|---|---|
| pfam13229 | Beta_helix | 1.61e-08 | 315 | 461 | 23 | 155 | Right handed beta helix region. This region contains a parallel beta helix region that shares some similarity with Pectate lyases. |
| cd00987 | PDZ_serine_protease | 1.38e-06 | 695 | 782 | 2 | 88 | PDZ domain of trypsin-like serine proteases, such as DegP/HtrA, which are oligomeric proteins involved in heat-shock response, chaperone function, and apoptosis. May be responsible for substrate recognition and/or binding, as most PDZ domains bind C-terminal polypeptides, though binding to internal (non-C-terminal) polypeptides and even to lipids has been demonstrated. In this subfamily of protease-associated PDZ domains a C-terminal beta-strand forms the peptide-binding groove base, a circular permutation with respect to PDZ domains found in Eumetazoan signaling proteins. |
| pfam13229 | Beta_helix | 3.50e-06 | 316 | 542 | 1 | 156 | Right handed beta helix region. This region contains a parallel beta helix region that shares some similarity with Pectate lyases. |
| COG0265 | DegQ | 4.59e-05 | 695 | 786 | 250 | 338 | Periplasmic serine protease, S1-C subfamily, contain C-terminal PDZ domain [Posttranslational modification, protein turnover, chaperones]. |
| pfam13229 | Beta_helix | 3.45e-04 | 423 | 568 | 1 | 138 | Right handed beta helix region. This region contains a parallel beta helix region that shares some similarity with Pectate lyases. |
CAZyme Hits help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End |
|---|---|---|---|---|---|
| AWW32784.1 | 3.77e-208 | 4 | 755 | 6 | 768 |
| BAU63437.1 | 2.48e-46 | 32 | 673 | 58 | 727 |
| AFZ35556.1 | 1.93e-40 | 46 | 665 | 71 | 711 |
| AWV97657.1 | 1.16e-35 | 32 | 665 | 30 | 709 |
| QWX84986.1 | 6.56e-34 | 3 | 665 | 1 | 710 |
PDB Hits download full data without filtering help
| Hit ID | E-Value | Query Start | Query End | Hit Start | Hit End | Description |
|---|---|---|---|---|---|---|
| 5MQP_A | 3.75e-16 | 30 | 453 | 26 | 501 | Glycosidehydrolase BT_1002 [Bacteroides thetaiotaomicron],5MQP_B Glycoside hydrolase BT_1002 [Bacteroides thetaiotaomicron],5MQP_C Glycoside hydrolase BT_1002 [Bacteroides thetaiotaomicron],5MQP_D Glycoside hydrolase BT_1002 [Bacteroides thetaiotaomicron],5MQP_E Glycoside hydrolase BT_1002 [Bacteroides thetaiotaomicron],5MQP_F Glycoside hydrolase BT_1002 [Bacteroides thetaiotaomicron],5MQP_G Glycoside hydrolase BT_1002 [Bacteroides thetaiotaomicron],5MQP_H Glycoside hydrolase BT_1002 [Bacteroides thetaiotaomicron] |
Swiss-Prot Hits help
SignalP and Lipop Annotations help
This protein is predicted as SP
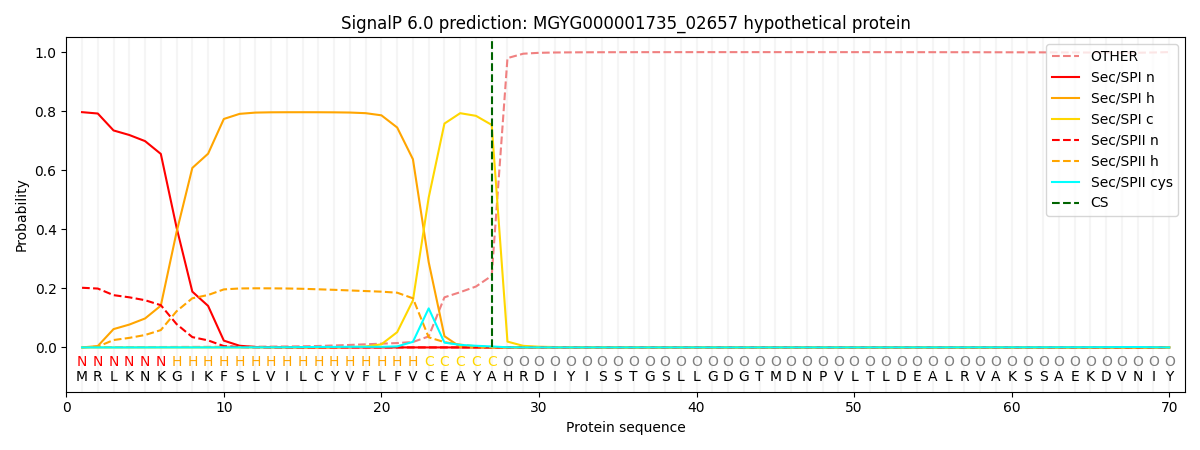
| Other | SP_Sec_SPI | LIPO_Sec_SPII | TAT_Tat_SPI | TATLIP_Sec_SPII | PILIN_Sec_SPIII |
|---|---|---|---|---|---|
| 0.003537 | 0.786116 | 0.209391 | 0.000343 | 0.000284 | 0.000300 |